ABSTRACT
Meta-analyses have been conducted with conflicting results on this topic. Due to missing several eligible studies in previous meta-analysis by Lam et al., we conducted an extensive systematic review and dose-response meta-analysis of randomized controlled trials in this regard. A comprehensive search was conducted across various databases, including MEDLINE/PubMed, ISI Web of Knowledge, Scopus, and Google Scholar, until November 2023. Based on the analysis of 33 studies comprising 2,047 individuals, it was found that there was a significant increase in body weight for each 1 g/day increase in omega-3 lipids (standardized MD [SMD], 0.52 kg; 95% confidence interval [CI], 0.31, 0.73; I2 = 95%; Grading of Recommendations Assessment, Development and Evaluation [GRADE] = low). Supplementation of omega-3 fatty acids did not yield a statistically significant impact on body mass index (BMI) (SMD, 0.12 kg/m2; 95% CI, −0.02, 0.27; I2 = 79%; GRADE = very low), lean body mass (LBM) (SMD, −0.02 kg; 95% CI, −0.43, 0.39; I2 = 97%; GRADE = very low), fat mass (SMD, 0.45 kg; 95% CI, −0.25, 1.15; I2 = 96%; GRADE = low), and body fat (SMD, 0.30%; 95% CI, −0.90, 1.51; I2 = 96%; GRADE = very low). After excluding 2 studies, the findings were significant for BMI. Regarding the results of the dose-response analysis, body weight increased proportionally by increasing the dose of omega-3 supplementation up to 4 g/day. Omega-3 fatty acid supplementation can improve body weight, but not BMI, LBM, fat mass, or body fat in cancer patients; large-scale randomized trials needed for more reliable results.
-
Trial Registration
-
Keywords: Meta-analysis; Fatty acids; Omega-3; Supplementation; Body weight; Randomized controlled trial
INTRODUCTION
Systemic inflammation is a defining characteristic of cancer, resulting in the generation of factors such as pro-inflammatory cytokines and metabolic tumor factors that contribute to metabolic changes and malnutrition. As the severity of the disease increases, cancer patients develop a condition called cachexia or sarcopenia, which is defined by fat-free mass reduction with or without fat mass reduction [
1]. Based on the document 40%–80% of patients with cancer have some signs of malnutrition, and 10%–20% of their deaths are associated with malnutrition [
2].
Previous studies suggest various interventions for cancer patients to gain lean body mass (LBM) and stabilize weight, including resistance exercises, nutritional supplements with high energy and protein, and hormonal drugs [
3]. However, some interventions, such as corticosteroids and progesterone agents, manifest side effects including shortness of breath, edema, and thromboembolic phenomena [
4]. Using enriched nutritional supplements seems easier for patients and has fewer complications and risks. Omega-3 fatty acids are bioactive nutrients predominantly present in seafood and vegetable oils. Eicosapentaenoic acid (EPA), alpha-linolenic acid, and docosahexaenoic acid (DHA) serve as instances of omega-3 fatty acids [
5]. The proposal of omega-3 fatty acid supplementation as a viable treatment for secondary complications of cancer has been made [
6]. Omega-3 fatty acids increase muscle power and weight by reduction of the pro-inflammatory cytokines [
7].
Some trials have implied that treatment with omega-3 fatty acids can stabilize weight and enhance LBM [
8], although studies related to weight gain and clinical results are inconsistent [
8]. Previous studies do not agree on the effective dose for positive effects in patients with cancer [
9]. A study reported a favorable impact on weight, body mass index (BMI), LBM, and overall survival [
10], while others did not indicate a statistically significant relation, and the evidence obtained was weak [
5].
Due to the inconsistency in the results of previous meta-analyses, not including several related studies in the previous meta-analyses, and also conducting several studies between the last meta-analysis and today, we decided to conduct this study. Also, findings from previous meta-analyses did not incorporate the use of Grading of Recommendations Assessment, Development and Evaluation)GRADE(tool to ascertain evidence certainty. Nor did they agree on the effective dose for these patients. Therefore, the purpose of this study was to use randomized controlled trials (RCTs) to examine the impact of various doses of omega-3 fatty acids on physical measurements in cancer patients.
MATERIALS AND METHODS
Systematic search
The registration code of our study protocol at PROSPERO is
CRD42023395341. We conducted comprehensive searches on MEDLINE/PubMed, Google Scholar, Web of Science, and Scopus for the released papers that appeared until November 2023.
Supplementary Table 1 illustrates the search strategy. No filter or limitation was used while searching the mentioned databases. All relevant reference lists of publications were reviewed to ensure no paper was missed. Two investigators (SMG and RAK) performed independent screening of the titles or abstracts and full-text publishing. If needed, disputes were settled through discussion or by the third investigator (HM).
Studies were included if they fulfilled the mentioned condition: 1) RCTs, with either parallel or cross-over design; 2) performed on participants with a different type of cancer (aged 18 years or older); 3) assessed the effect of treatment with omega-3 fatty acids, regardless of its source (EPA, DHA, both and other); 4) had considered at least one of the following variables as the outcome: body weight, LBM, BMI, body fat, and fat mass; and 5) articles that presented means and standard deviations (SDs) of changes in interest-related outcomes, or provided details that allowed you to determine those values. Studies were excluded if: 1) they were performed on adolescents (under 18 years of age) and lactating and pregnant women; and 2) they investigated any other intervention plus omega-3 fatty acids.
Data extraction
Two independent reviewers (SMG and SZM) obtained information from the included studies. The information extracted from each eligible trial includes the first author’s name, publication year, study location, study design (parallel or cross-over), patient numbers in the omega-3 fatty acids and control groups, cancer type, duration of therapy, dosage, type of intervention and control, concurrent treatment, mean age, mean baseline BMI, and drop out. Data reported in interquartile ranges and standard errors (SEs) were converted to SDs. Some anthropometric indicators have been converted from different units to the most commonly used units. Figures and charts in the original papers were quantified using web plot digitizer software.
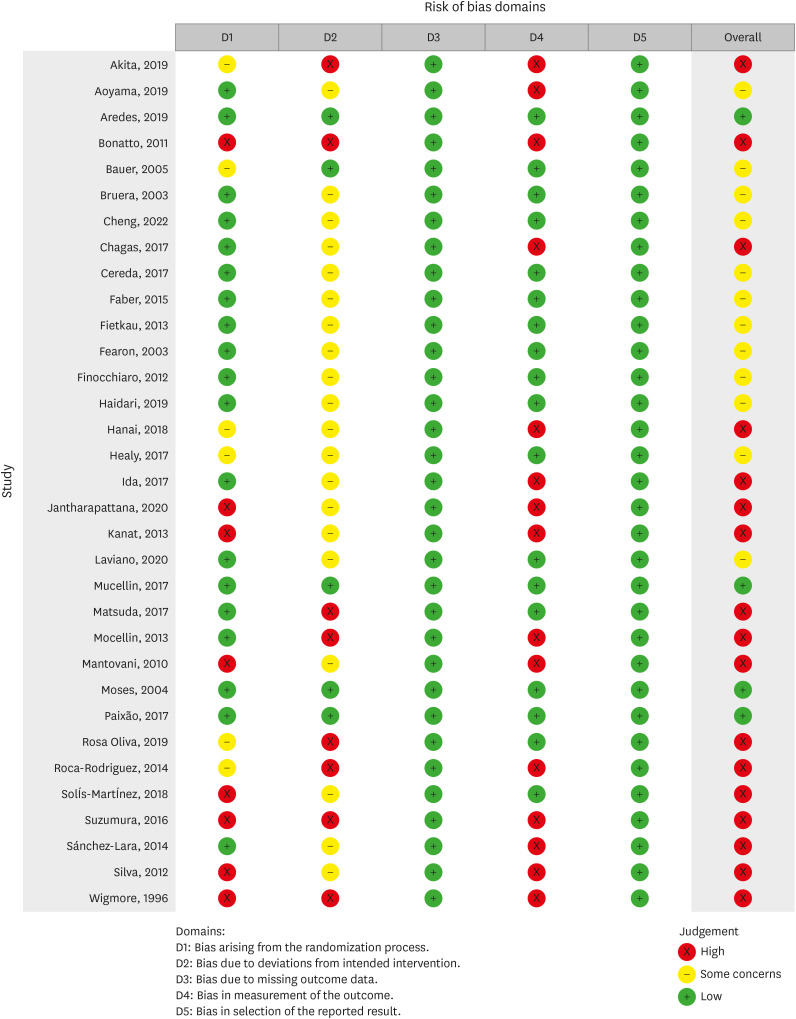
Risk of bias (quality) assessment
Two reviewers (SMG and SZM) evaluated the quality of the selected trials by the Cochrane risk of bias tool (RoB 2) [
11]. It comprises five domains of bias including 1) the procedure of randomization; 2) shifts from the aimed treatments; 3) lacking outcome information; 4) outcome measurement; and 5) the reported results selection. Based on the Cochrane Handbook recommendation, studies were classified as “low risk of bias,” “some concerns,” or “high risk of bias” with respect to each domain (
Figures 1 and
2).
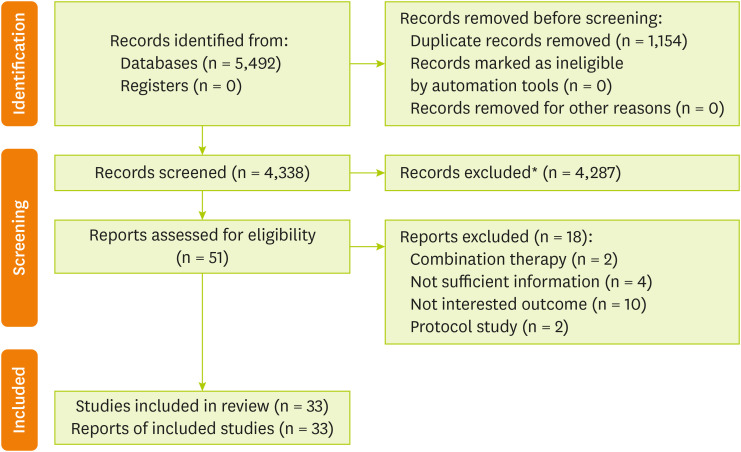
Figure 1
Literature search and study selection process.
*Articles removed based on title and abstract.
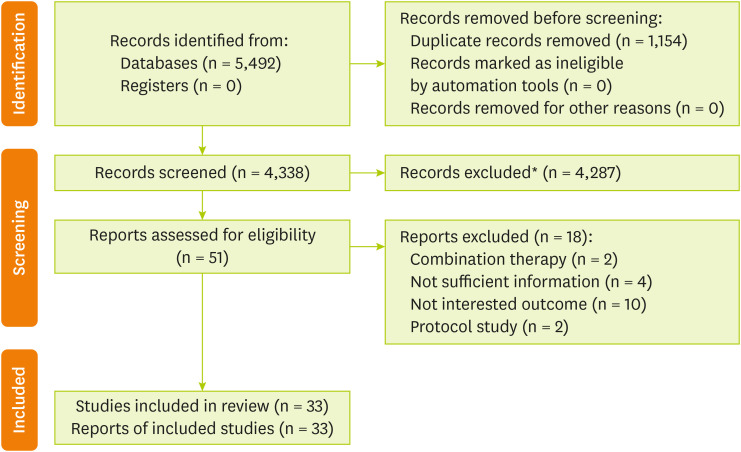
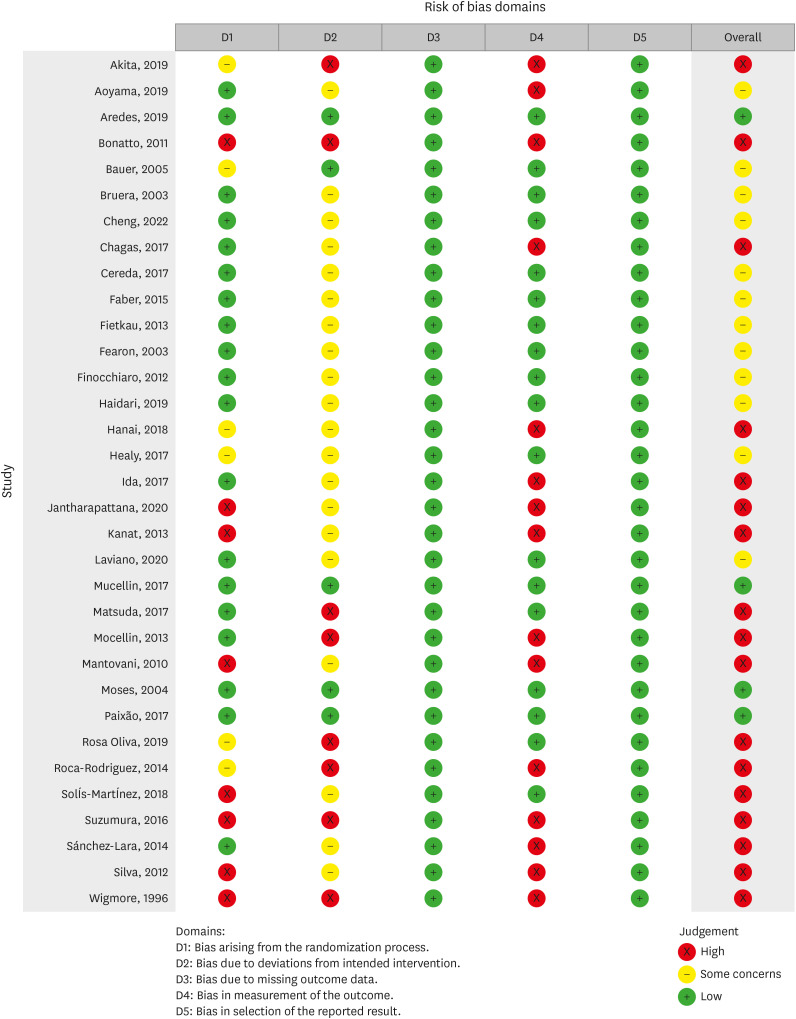
Figure 2Risk of bias graph for included studies.
Statistical analysis
To describe the findings of the current comprehensive study, we used the standardized mean difference (SMD) and 95% confidence intervals (CIs) of the change regarding BMI, body weight, LBM, body fat, and fat mass in the omega-3 lipids supplementing group as opposed to the controls. The first step was calculating the change in body weight, BMI, LBM, fat mass, and body fat from baseline in each study arm. The Cochrane Handbook guides calculating mean and SD values for studies lacking reported mean changes and SD changes [
11]. In cases where trials reported SEs rather than SDs, we converted the SEs to SDs. In the case of a lack of SD or SE, our meta-analysis utilized the mean SDs from other eligible studies. Second, we calculated SMD and the SE of change in BMI, body weight, LBM, body fat, and fat mass based on the method provided by Crippa and Orsini [
12] for each 1 g per day increase in omega-3 fatty acids in the omega-3 fatty acids supplementation group as opposed to the controls in each trial. To apply this technique, we must have the mean and associated SD of change in weight, BMI, LBM, fat mass, and body fat for each trial arm, along with the individual number in each arm. It also needs the dosage (grams per day) of omega-3 fatty acids in each study arm. A random-effects model was utilized to pool the trial-specific results. As a next step, we performed predetermined subgroup analyses according to the kind of omega-3 fatty acids, present treatment, duration of intervention, cancer site, quality of studies, route of administration, adherence, weight status, quality of studies, and weight status. The p value for subgroup differences was calculated by meta-regression analysis. An influence analysis was undertaken to define how each study might affect the main findings. Publication bias was measured through Egger’s and Begg’s tests. The I
2 statistic was used for evaluating heterogeneity, and the χ2 test was conducted for homogeneity (Pheterogeneity > 0.10).
The effect of each increase of 1 g/day in omega-3 fatty acids on body weight, BMI, LBM, fat mass, and body fat was examined in a dose–response meta-analysis. In order to measure the risk difference, odds ratio (OR), and 95% CIs, we used the participant’s number and events in the omega-3 fatty acids supplementation group and controls. Stata 17.0 was used to carry out the statistical analyses. A p value of less than 0.05, with two tails, was deemed to be statistically significant.
Grading the evidence
A GRADE [
13] method was utilized to assess the certainty of the evidence. Evidence can be grouped as high, moderate, low, or very low in terms of certainty using the GRADE approach. When we found large effect sizes or a gradient in dose response, the certainty of evidence increased.
RESULTS
Study selection
As revealed in
Figure 1, a total of 5,492 records were found from searches of databases and reference lists, and after screening the titles/abstracts, 4,287 irrelevant papers were excluded. Evaluation of 51 full-text articles contributed to identifying 33 RCTs becoming eligible for entering this meta-analysis [
10,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43,
44,
45]. Reasons for exclusion were deemed prescribed omega-3 fatty acids as a combination treatment (n = 2), articles with no sufficient information (n = 4), articles that included outcomes that were not interesting for our study (n = 10), and protocol studies (n = 2) (
Supplementary Table 2).
Supplementary Table 3 and Supplementary References abbreviated the main characteristics of 33 RCTs, including 2,047 patients with cancer, among which 1,008 and 1,039 patients were in the omega-3 fatty acids group and controls, respectively. Included RCTs were published between 1996 and 2022. These RCTs were done in Brazil [
16,
18,
21,
36,
37,
39,
42,
44], UK [
24,
38,
45], Japan [
14,
15,
22,
28,
35], China [
10], Iran [
27], Thailand [
31], Mexico [
22,
41,
43], Spain [
40], Italy [
20,
26,
34], Australia [
17], Canada [
19], Ireland [
29], Netherland [
23], Germany [
25], Turkey [
32], and four countries: Croatia, Italy, Slovakia, and Sweden [
33]. All studies other than one study had parallel study designs [
45]. The average age of patients spanned between 45.14 years and 68 years. The kind of omega-3 lipids was EPA in nine trials [
17,
24,
28,
29,
30,
31,
32,
34,
38], and the additional 24 used EPA plus DHA [
10,
14,
15,
16,
18,
19,
20,
21,
22,
23,
25,
26,
27,
33,
35,
36,
37,
39,
40,
41,
42,
43,
44,
45]. The route of administration was oral, enteral, and oral/enteral in 28 [
10,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
26,
27,
28,
30,
31,
32,
33,
34,
36,
37,
38,
39,
41,
42,
44,
45], three [
25,
29,
35], and two RCTs [
40,
43], respectively. The duration of supplementation was between 2 and 24 weeks. The length of supplementation was ≤ 8 weeks in 19 RCTs [
14,
16,
17,
18,
23,
24,
25,
26,
27,
28,
29,
30,
31,
35,
38,
39,
41,
43,
44] and > 8 weeks in 14 RCTs [
10,
15,
19,
20,
21,
22,
32,
33,
34,
36,
37,
40,
42,
45]. Four trials were demonstrated to have a low risk of bias [
16,
36,
38,
39], 12 RCTs were shown to have some concerns [
10,
15,
17,
19,
20,
23,
24,
25,
26,
27,
29,
33], and the other 17 RCTs had a high risk of bias (
Figures 2 and
3) [
14,
18,
21,
22,
28,
29,
30,
31,
32,
34,
35,
37,
40,
41,
42,
43,
44,
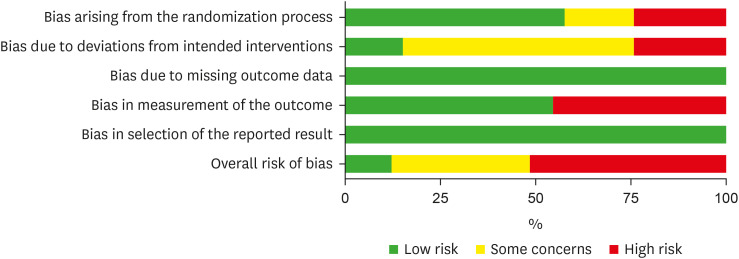
45].
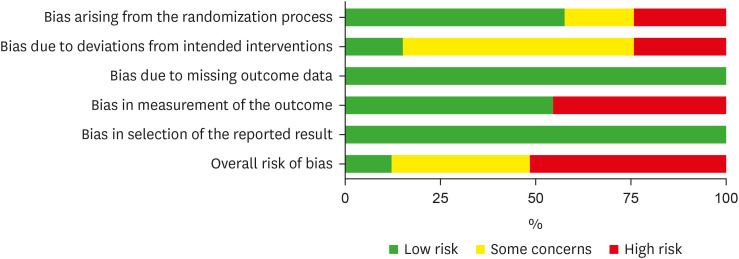
Figure 3Risk of bias summary for included studies.
Omega-3 lipids and body weight
The compiled effect estimates of 28 RCTs (1,735 participants) [
10,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
35,
36,
37,
38,
39,
41,
42,
43,
45] revealed that every 1 g/day rise in the omega-3 lipids significantly incremented the weight (SMD, 0.52 kg; 95% CI, 0.31, 0.73; I
2 = 95%) (
Supplementary Figure 1,
Table 1). The main finding continued to be significant and spanned between 0.44 (0.24, 0.64) and 0.58 (0.37, 0.78) after the exclusion of each trial step-by-step from the principal analysis.
Table 1The effectiveness of omega-3 fatty acids supplementation on anthropometric variables and adverse events in cancer patients
Table 1
|
Outcome(s) |
No. of trials (participants) |
Type of effect size |
Effect size (95% CI) |
p value |
I2, pheterogeneity
|
GRADE certainty |
|
Weight (kg) |
28 (1,735) |
SMD |
0.52 (0.31, 0.73) |
< 0.001 |
95%, < 0.001 |
Low |
|
BMI (kg/m2) |
17 (849) |
SMD |
0.12 (−0.02, 0.27) |
0.09 |
79%, < 0.001 |
Very low |
|
Lean body mass (kg) |
13 (1,011) |
SMD |
−0.02 (−0.43, 0.39) |
0.91 |
97%, < 0.001 |
Very low |
|
Fat mass (kg) |
4 (345) |
SMD |
0.45 (−0.25, 1.15) |
0.21 |
96%, < 0.001 |
Low |
|
Body fat (%) |
3 (101) |
SMD |
0.30 (−0.90, 1.51) |
0.62 |
96%, < 0.001 |
Very low |
|
Adverse event |
12 (811) |
OR |
1.06 (0.84, 1.34) |
0.61 |
38%, 0.08 |
Low |
|
Adverse event |
12 (811) |
RD |
0.02 (−0.02, 0.07) |
0.33 |
55%, 0.01 |
Low |
In the subgroup analyses, the impact of omega-3 lipids on body weight continued to be notable in all subgroups (
Supplementary Table 4). The findings remained substantial in trials treated with EPA and DHA (SMD, 0.69 kg; 95% CI, 0.43, 0.94; I
2 = 95%; n = 22), included participants with normal body weight (SMD, 1.09 kg; 95% CI, 0.60, 1.58; I
2 = 96%; n = 11), as well as in studies in which the length of supplementation was ≤ 8 weeks (SMD, 0.50 kg; 95% CI, 0.29, 0.71; I
2 = 92%; n = 18). We identified no considerable publication bias (p
Egger = 0.79, p
Begg = 0.06, or observation of the funnel plot;
Supplementary Figure 2).
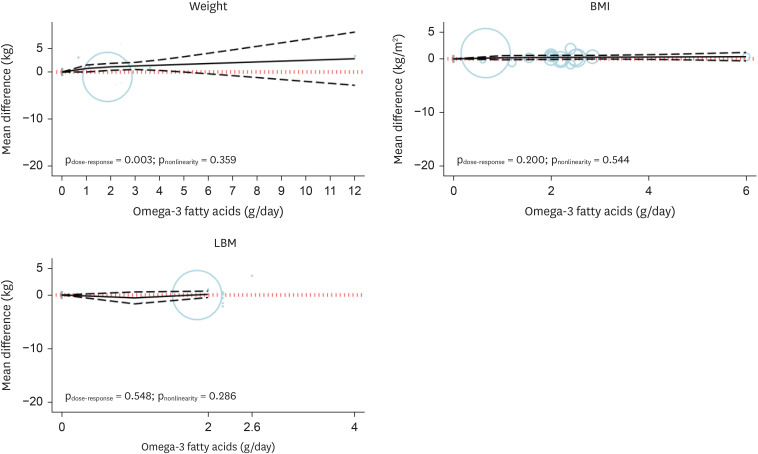
The non-linear dose-dependent analysis was performed for all trials. The findings revealed that the body weight raised proportionally with the raising in omega-3 fatty acids supplement at most a level of 4 g/day (SMD
4 g/day, 1.45 kg; 95% CI, 0.33, 2.58), with no considerable alteration in effect estimate at higher doses of omega-3 fatty acids supplementation (p
nonlinearity = 0.359, p
dose-response = 0.003;
Figure 4,
Table 2).
Figure 4
Dose-dependent effect of omega-3 fatty acids supplementation on body weight, BMI, and lean body mass. Solid lines represent standardized mean difference, and dashed lines represent 95% confidence interval.
BMI, body mass index; LBM, lean body mass.

Table 2The effects of different doses of omega-3 fatty supplementation acids on anthropometric variables in cancer patients from the nonlinear dose-response meta-analysis (standardized mean difference and 95% confidence interval)
Table 2
|
Omega-3 supplementation (g/day) |
0 (ref) |
1 |
1.5 |
2 |
2.5 |
3 |
4 |
6 |
8 |
10 |
12 |
GRADE |
|
Weight (all trials) (kg) |
0 |
0.75 (0.01, 1.49)
|
0.96 (0.17, 1.75)
|
1.10 (0.36, 1.84)
|
1.20 (0.50, 1.89)
|
1.28 (0.53, 2.04)
|
1.45 (0.33, 2.58)
|
1.80 (−0.39, 3.98) |
2.14 (−1.19, 5.47) |
2.48 (−2.01, 6.98) |
2.83 (−2.84, 8.49) |
Low |
|
BMI (all trial), (kg/m2) |
0 |
0.21 (−0.18, 0.59) |
0.25 (−0.16, 0.67) |
0.28 (−0.10, 0.66) |
0.30 (−0.05, 0.46) |
0.31 (−0.03, 0.65) |
0.34 (−0.09, 0.77) |
0.40 (−0.38, 1.19) |
- |
- |
- |
Very low |
|
LBM (all trails), (kg) |
0 |
−0.55 (−1.67, 0.57) |
−0.31 (−1.11, 0.50) |
0.12 (−0.48, 0.72) |
0.60 (−0.59, 1.79) |
- |
- |
- |
- |
- |
- |
Very low |
Omega-3 lipids and BMI
The compiled impact estimate of 17 RCTs (849 participants) [
10,
14,
16,
21,
22,
25,
27,
29,
32,
33,
36,
37,
39,
40,
42,
43,
44] revealed that every 1 g/day increase in the omega-3 fats did not have notable influence on BMI (SMD, 0.12 kg/m
2; 95% CI, −0.02, 0.27; I
2 = 79%;
Supplementary Figure 3,
Table 1). The sensitivity analysis revealed that the final result was heavily affected by two trials [
22] (SMD, 0.16; 95% CI, 0.03, 0.30) and Suzumura et al. [
44] (SMD, 0.15; 95% CI, 0.01, 0.29). When these trials were eliminated, we observed a considerable impact of omega-3 fatty acids on BMI.
In the subgroup analyses, we found no significant effect on BMI in all subgroups after supplementation with the wide range of daily dosages of omega-3 fatty acids. However, we identified a trivial considerable improvement in BMI after omega-3 fatty acids supplementation in trials included normal weight (SMD, 0.34 kg/m
2; 95% CI, 0.07, 0.61; I
2 = 83%; n = 7) and head and neck cancer patients (SMD, 0.16 kg/m
2; 95% CI, 0.01, 0.31; I
2 = 48%; n = 3), as well as those with some concerns risk of bias (SMD, 0.32 kg/m
2; 95% CI, 0.01, 0.62; I
2 = 88%; n = 6) (
Supplementary Table 5). We identified no considerable publication bias (p
Egger = 0.90, p
Begg = 0.59, or observation of the funnel plot;
Supplementary Figure 4).
The non-linear dose-dependent analysis was conducted for all trials. Our findings revealed that there was no dose-dependent effect on BMI as a portion of omega-3 lipids supplement increased (p
nonlinearity = 0.544, p
dose-response = 0.200;
Figure 4,
Table 2).
The compiled impact estimate of 13 RCTs, including 1,011 participants [
15,
17,
24,
28,
29,
32,
34,
35,
37,
38,
39,
41,
43], indicated that every 1 g/day increase in the omega-3 fats had no notable influence on LBM (SMD, −0.02 kg; 95% CI, −0.43, 0.39; I
2 = 97%;
Supplementary Figure 5,
Table 1). The findings of sensitivity analysis showed no alteration in results when individual trials were omitted step by step (spanned between −0.15 [−0.51, 0.21] and 0.09 [−0.31, 0.49]). We identified no considerable publication bias (p
Egger = 0.51, p
Begg = 0.18, or observation of the funnel plot;
Supplementary Figure 6).
In the subgroup analysis conducted across the wide range of daily dosages of omega-3 fatty acids, the impact of omega-3 fatty acids on LBM remained insignificant for all subgroups. However, trials conducted on patients undergoing chemotherapy or surgery and all therapies showed a significant improvement in LBM after omega-3 fatty acids supplementation. Moreover, trials conducted on patients with head and neck, breast, and lung cancer revealed a favorable impact (
Supplementary Table 6).
The non-linear analysis in different doses was performed for all trials. The findings revealed no dose-dependent effect on LBM when the portion of omega-3 lipids was increased (p
nonlinearity = 0.286, p
dose-response = 0.548;
Figure 4,
Table 2).
The overall results of 4 RCTs (345 participants) [
22,
29,
39,
43] revealed that every 1 g/day increase in the omega-3 lipids did not have a considerable impact on fat mass (SMD, 0.45 kg; 95% CI, −0.25, 1.15; I
2 = 96%;
Supplementary Figure 7,
Table 1).
Three RCTs were analyzed, involving 101 participants [
22,
37,
39], increasing omega-3 fatty acids by 1 g/day had no significant impact on body fat (SMD, 0.30%; 95% CI, −0.90, 1.51; I
2 = 96%;
Supplementary Figure 8,
Table 2).
The quality of the evidence for body weight, BMI, LBM, fat mass, and body fat percentage varies from low to very low for the trials based on the GRADE tool (
Supplementary Table 7). Body weight and fat mass were shown to have low certainty of the evidence for the downgrade of inconsistency and imprecision. BMI, LBM, and body fat percentage were revealed to have a very low certainty through degradation of the risk of bias, inconsistency, and imprecision.
Twelve RCTs reported adverse events throughout the intervention duration [
14,
16,
19,
20,
22,
24,
25,
32,
33,
34,
35,
39]. We found an increment in adverse events by 2 per 100 subjects with cancer (risk difference, 0.02; 95% CI, −0.02, 0.07; OR, 1.06; 95% CI, 0.84, 1.34; GRADE = low) (
Supplementary Figures 9 and
10,
Table 1).
DISCUSSION
In our current comprehensive review and dose-response meta-analysis of RCTs, we looked at 33 studies' findings on the therapeutic and dietary advantages of omega-3 fatty acids in patients with cancer (including 2,047 patients). A key observation is that there is a promising impact on body weight with every 1 g/day rise in the intake of omega-3 fatty acids relative to the control group. However, each 1 g/day rise in omega-3 fats demonstrated no statistically significant impact on BMI, LBM, fat mass, and body fat. Moreover, although we found no significant impact on BMI in the main analysis, the elimination of the two trials [
22] (SMD, 0.16) and Suzumura et al. [
44] (SMD, 0.15) revealed a favorable impact on BMI.
The study by de la Rosa Oliva et al. [
22] indicated that patients were already screened to ensure an enhanced profile preference, which could bias the results, not manifesting the advantages of "common and current" patients. In another study [
44], the fish oil intake was comparatively low, which could have led to some results that were insignificant. Plasma or erythrocytes should also have been measured for omega-3 polyunsaturated fatty acid. Furthermore, the low number of participants in both studies, as well as a high risk of bias (based on RoB-2), may result in major differences between them and comparative studies. According to a meta-analysis, body weight showed a proportional increase with omega-3 fats supplementation up to 4 g/day. Although our findings revealed no dose-dependent impact on BMI, LBM, fat mass, and body fat when increasing the dosage of omega-3 fatty acids supplement (GRADE = low to very low), we discovered compelling evidence of a substantial effect on body weight through our prespecified subgroup analyses. This effect was observed in patients treated with EPA & DHA, having normal body weight, and a supplementation duration of ≤ 8 weeks. In addition, supplementing with omega-3 fats indicated a trivial increase in the BMI in trials that included normal weight and patients with head and neck cancer, as well as those with some concerns risk of bias. These results can have an important effect on the clinical use of omega-3 fatty acids.
The difference in the effect of omega-3 fatty acids on anthropometric indicators in different types of cancer can be attributed to several factors: 1) Inflammatory markers: different types of cancer may have varying levels of inflammatory markers, which can affect how omega-3 fatty acids modulate the inflammatory response. For example, studies have shown differences in interleukin (IL)-6 levels between lung and gastric cancer patients [
10], 2) Tumor-specific factors: the biological characteristics of different tumor types may influence how they respond to omega-3 supplementation, potentially affecting nutritional outcomes [
46], and 3) Baseline nutritional status: the initial nutritional status of patients can influence how they respond to omega-3 supplementation. Patients with more severe malnutrition at baseline may show more significant improvements [
46].
We need to consider several factors that could explain the apparent discrepancy between the lack of impact on fat mass and lean mass, yet an increase in body weight from omega-3 supplementation in cancer patients. These reasons can include: 1) Fluid retention: omega-3 fatty acids, particularly EPA and DHA, may influence fluid balance in the body. This could lead to increased water retention, which would contribute to higher body weight without necessarily affecting fat or lean mass measurements [
10], 2) Edema: cancer patients often experience edema due to various factors, including inflammation and treatment side effects. Omega-3 supplementation might modulate inflammatory responses, potentially affecting fluid distribution in the body and leading to weight gain without changes in fat or lean mass [
10], 3) Measurement limitations: some anthropometric measurement techniques may not be sensitive enough to detect small changes in body composition, especially in cancer patients who may have altered tissue characteristics. This could result in apparent weight gain without detectable changes in fat or lean mass [
10], and 4) Interaction with cancer treatments: the effects of omega-3 supplementation on body weight might be influenced by concurrent cancer treatments, which could mask or alter the impact on specific body composition components [
10].
Despite the results of the current study, a previous meta-analysis showed a non-significant impact of omega-3 fatty acids supplementation on body weight. A meta-analysis [
47] of 31 randomized trials revealed that omega-3 fatty acids supplementation does not enhance muscle maintenance (mean difference, −0.47 kg; 95% CI, −2.17, 1.24; n = 505) or body weight (mean difference, 0.98 kg; 95% CI, −1.00, 2.96; n = 839) in patients with cancer. To examine the impact of omega-3 fats supplementing on body weight, ten additional trials were incorporated, which were not previously considered in the meta-analysis.
Another important concern about meta-analysis was the use of final values instead of changes from baselines, which failed to consider differences at baseline between studies, potentially influencing the pooled results. In addition, our findings were aligned with the meta-analysis conducted in 2022 [
48]. The study's results reveal that omega-3 fatty acids had a nonsignificant impact on LBM (SMD, 0.17; 95% CI, −0.01, 0.35) and BMI (SMD, 0.06; 95% CI, −0.16, 0.27). However, body weight showed statistical significance (SMD, 0.26; 95% CI, 0.06, 0.45).
Ma et al. [
49] revealed that there was a significant gain in body weight (WMD, 0.62 kg; 95% CI, 0.54, 0.69) and LBM (WMD, 0.96 kg; 95% CI, 0.86, 1.06) in participants with pancreatic cancer with unresectable tumors were compared based on their consumption of an omega-3 fats enriched oral nutritional supplements versus conventional nutrition. Moreover, some newly published trials were missing in all previous meta-analyses. We included 10 additional studies; none mentioned the sensitivity analysis results for the BMI variable assessed in our study. In the present study, investigating two new variables (body fat and fat mass), evaluating the patients with a different type of cancer, and performing a dose-response analysis can help to examine the impact of omega-3 fatty acids supplementation on anthropometric variables more accurately.
Some potential biochemical processes could account for the advantages of omega-3 fatty acids supplementation in patients with cancer. Omega-3 fatty acids, particularly EPA, bring about various effects through several mechanisms. These include reducing catabolic activity in protein degradation and lipid mobilization pathways, as well as decreasing glucose consumption in skeletal muscle [
44,
45,
46,
47,
48,
49]. In other words, Studies suggest that this nutrient suppresses lipogenesis, reduces free fatty acid deposition in muscles, and stimulates their oxidation [
44,
45,
46,
47,
48,
49]. Attenuating side effects are mainly achieved by modulating inflammation (lessening the generation of pro-inflammatory cytokines including IL-6, IL-1, and tumor necrosis factor-α and tumor factors) and inhibiting the synthesis of proteolysis-inducing factors, thereby reducing skeletal muscle proteolysis [
7,
44,
45,
46,
47,
48,
49].
Several strengths can be found in this review. An assessment of all published studies on the impact of omega-3 fatty acids on anthropometric variables. We also included a wide range of patients with cancer. The current meta-analysis offered different insights about the impact of omega-3 lipids supplementing in different doses on anthropometric variables in individuals with cancer that were not given in the prior meta-analyses [
44,
45,
46,
47,
48,
49]. GRADE was used to assess the certainty of evidence.
In interpreting the findings of the present meta-analysis, it is essential to consider the following limitations. First, the findings may be limited in generalizability due to high statistical heterogeneity. Although we conducted subgroup analysis based on different variables, we could not determine the source of heterogeneity. Another constraint is the limited sample size observed in certain trials incorporated within the current study, along with the absence of outcome assessment blinding and allocation concealment in certain trials. The findings from trials with low bias risk were consistent with the main analysis. Additionally, various discrepancies between trials, such as cancer duration, severity, medication, and ω-3 supplement quality, can lead to notable variances. Finally, the quality of the studies was low to very low, so the power of our review was further reduced.
CONCLUSION
The present dose-response meta-analysis of 33 RCTs showed that each 1 g/day rise in omega-3 fatty acids could favorably affect body weight with the greatest impact on the dosage of 4 g/day. However, this result was insignificant for BMI, LBM, body fat, and fat mass., The findings were significant for BMI, even though two studies were excluded. Furthermore, the overall quality of evidence was ranked low to very low, indicating that to examine the impact of omega-3 fatty acid supplementation on anthropometric factors in cancer patients, additional well-designed studies are needed.
Tehran University of Medical Sciences and Health Serviceshttps://doi.org/10.13039/501100004484
65329
NOTES
-
Funding: The funding for this study was provided by a grant from Tehran University of Medical Sciences, Tehran, Iran (registration number: 65329).
-
Conflict of Interest: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Ghoreishy SM, Mohammadi H.
Data curation: Ghoreishy SM, Zeraattalab-Motlagh S, Amiri Khosroshahi R, Noormohammadi M, Mohammadi H.
Formal analysis: Ghoreishy SM, Zeraattalab-Motlagh S.
Writing - original draft: Ghoreishy SM, Zeraattalab-Motlagh S, Amiri Khosroshahi R, Hemmati A, Noormohammadi M, Mohammadi H.
Writing - review & editing: Ghoreishy SM, Noormohammadi M, Mohammadi H.
SUPPLEMENTARY MATERIALS
Supplementary Table 1
Detailed search strategy in each database, last updated Nov 2023
cnr-13-186-s001.xls
Supplementary Table 3
Characteristics of studies included in the meta-analysis of the effect of omega-3 fatty acids supplementation on anthropometric variables
cnr-13-186-s003.xls
Supplementary Table 4
Subgroup analysis of the effect of omega-3 fatty acids supplementation on body weight
cnr-13-186-s004.xls
Supplementary Table 5
Subgroup analysis of the effect of omega-3 fatty acids supplementation on BMI
cnr-13-186-s005.xls
Supplementary Table 6
Subgroup analysis of the effect of omega-3 fatty acids supplementation on LBM
cnr-13-186-s006.xls
Supplementary Table 7
GRADE evidence table for the effect of omega-3 fatty acids supplementation o anthropometric variables
cnr-13-186-s007.xls
Supplementary Figure 1
SMD of body weight for a 1 g/day increment in omega-3 fatty acids supplementation using random effects model.
cnr-13-186-s008.ppt
Supplementary Figure 2
The funnel plot of omega-3 fatty acids (each 1 g/day) supplementation on body weight.
cnr-13-186-s009.ppt
Supplementary Figure 3
SMD of body mass index for a 1 g/day increment in omega-3 fatty acids supplementation using random effects model.
cnr-13-186-s010.ppt
Supplementary Figure 4
The funnel plot of omega-3 fatty acids (each 1 g/day) supplementation on body mass index.
cnr-13-186-s011.ppt
Supplementary Figure 5
SMD of lean body mass for a 1 g/day increment in omega-3 fatty acids supplementation using random effects model.
cnr-13-186-s012.ppt
Supplementary Figure 6
The funnel plot of omega-3 fatty acids (each 1 g/day) supplementation on lean body mass.
cnr-13-186-s013.ppt
Supplementary Figure 7
SMD of fat mass for a 1 g/day increment in omega-3 fatty acids supplementation using random effects model.
cnr-13-186-s014.ppt
Supplementary Figure 8
SMD of body fat percentage for a 1 g/day increment in omega-3 fatty acids supplementation using random effects model.
cnr-13-186-s015.ppt
Supplementary Figure 9
OR of adverse events for a 1 g/day increment in omega-3 fatty acids supplementation using random effects model.
cnr-13-186-s016.ppt
Supplementary Figure 10
Risk difference of adverse events for a 1 g/day increment in omega-3 fatty acids supplementation using random effects model.
cnr-13-186-s017.ppt
REFERENCES
- 1. Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr 2017;36:1187-1196.
- 2. Wang Y, Zhang T, Liu R, Chang M, Wei W, et al. New perspective toward nutritional support for malnourished cancer patients: role of lipids. Compr Rev Food Sci Food Saf 2021;20:1381-1421.
- 3. Tsai S. Importance of lean body mass in the oncologic patient. Nutr Clin Pract 2012;27:593-598.
- 4. Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11-48.
- 5. Dewey A, Baughan C, Dean T, Higgins B, Johnson I. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst Rev 2007;2007:CD004597.
- 6. Freitas RDS, Campos MM. Protective effects of omega-3 fatty acids in cancer-related complications. Nutrients 2019;11:945.
- 7. Huang YH, Chiu WC, Hsu YP, Lo YL, Wang YH. Effects of omega-3 fatty acids on muscle mass, muscle strength and muscle performance among the elderly: a meta-analysis. Nutrients 2020;12:3739.
- 8. Werner K, Küllenberg de Gaudry D, Taylor LA, Keck T, Unger C, et al. Dietary supplementation with n-3-fatty acids in patients with pancreatic cancer and cachexia: marine phospholipids versus fish oil - a randomized controlled double-blind trial. Lipids Health Dis 2017;16:104.
- 9. Gorjao R, Dos Santos CMM, Serdan TDA, Diniz VLS, Alba-Loureiro TC, et al. New insights on the regulation of cancer cachexia by N-3 polyunsaturated fatty acids. Pharmacol Ther 2019;196:117-134.
- 10. Cheng M, Zhang S, Ning C, Huo Q. Omega-3 fatty acids supplementation improve nutritional status and inflammatory response in patients with lung cancer: a randomized clinical trial. Front Nutr 2021;8:686752.
- 11. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, et al. Cochrane handbook for systematic reviews of interventions. Hoboken: John Wiley & Sons; 2019.
- 12. Crippa A, Orsini N. Dose-response meta-analysis of differences in means. BMC Med Res Methodol 2016;16:91.
- 13. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383-394.
- 14. Akita H, Takahashi H, Asukai K, Tomokuni A, Wada H, et al. The utility of nutritional supportive care with an eicosapentaenoic acid (EPA)-enriched nutrition agent during pre-operative chemoradiotherapy for pancreatic cancer: prospective randomized control study. Clin Nutr ESPEN 2019;33:148-153.
- 15. Aoyama T, Yoshikawa T, Ida S, Cho H, Sakamaki K, et al. Effects of perioperative eicosapentaenoic acid-enriched oral nutritional supplement on lean body mass after total gastrectomy for gastric cancer. J Cancer 2019;10:1070-1076.
- 16. Aredes MA, da Camara AO, de Paula NS, Fraga KYD, do Carmo MDGT, et al. Efficacy of ω-3 supplementation on nutritional status, skeletal muscle, and chemoradiotherapy toxicity in cervical cancer patients: a randomized, triple-blind, clinical trial conducted in a middle-income country. Nutrition 2019;67-68:110528.
- 17. Bauer J, Capra S, Battistutta D, Davidson W, Ash S, et al. Compliance with nutrition prescription improves outcomes in patients with unresectable pancreatic cancer. Clin Nutr 2005;24:998-1004.
- 18. Bonatto SJ, Oliveira HH, Nunes EA, Pequito D, Iagher F, et al. Fish oil supplementation improves neutrophil function during cancer chemotherapy. Lipids 2012;47:383-389.
- 19. Bruera E, Strasser F, Palmer JL, Willey J, Calder K, et al. Effect of fish oil on appetite and other symptoms in patients with advanced cancer and anorexia/cachexia: a double-blind, placebo-controlled study. J Clin Oncol 2003;21:129-134.
- 20. Cereda E, Cappello S, Colombo S, Klersy C, Imarisio I, et al. Nutritional counseling with or without systematic use of oral nutritional supplements in head and neck cancer patients undergoing radiotherapy. Radiother Oncol 2018;126:81-88.
- 21. Chagas TR, Borges DS, de Oliveira PF, Mocellin MC, Barbosa AM, et al. Oral fish oil positively influences nutritional-inflammatory risk in patients with haematological malignancies during chemotherapy with an impact on long-term survival: a randomised clinical trial. J Hum Nutr Diet 2017;30:681-692.
- 22. de la Rosa Oliva F, Meneses García A, Ruiz Calzada H, Astudillo de la Vega H, Bargalló Rocha E, et al. Effects of omega-3 fatty acids supplementation on neoadjuvant chemotherapy-induced toxicity in patients with locally advanced breast cancer: a randomized, controlled, double-blinded clinical trial. Nutr Hosp 2019;36:769-776.
- 23. Faber J, Uitdehaag MJ, Spaander M, van Steenbergen-Langeveld S, Vos P, et al. Improved body weight and performance status and reduced serum PGE2 levels after nutritional intervention with a specific medical food in newly diagnosed patients with esophageal cancer or adenocarcinoma of the gastro-esophageal junction. J Cachexia Sarcopenia Muscle 2015;6:32-44.
- 24. Fearon KCH, Von Meyenfeldt MF, Moses AGW, Van Geenen R, Roy A, et al. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut 2003;52:1479-1486.
- 25. Fietkau R, Lewitzki V, Kuhnt T, Hölscher T, Hess CF, et al. A disease-specific enteral nutrition formula improves nutritional status and functional performance in patients with head and neck and esophageal cancer undergoing chemoradiotherapy: results of a randomized, controlled, multicenter trial. Cancer 2013;119:3343-3353.
- 26. Finocchiaro C, Segre O, Fadda M, Monge T, Scigliano M, et al. Effect of n-3 fatty acids on patients with advanced lung cancer: a double-blind, placebo-controlled study. Br J Nutr 2012;108:327-333.
- 27. Haidari F, Abiri B, Iravani M, Ahmadi-Angali K, Vafa M. Randomized study of the effect of vitamin D and omega-3 fatty acids cosupplementation as adjuvant chemotherapy on inflammation and nutritional status in colorectal cancer patients. J Diet Suppl 2020;17:384-400.
- 28. Hanai N, Terada H, Hirakawa H, Suzuki H, Nishikawa D, et al. Prospective randomized investigation implementing immunonutritional therapy using a nutritional supplement with a high blend ratio of ω-3 fatty acids during the perioperative period for head and neck carcinomas. Jpn J Clin Oncol 2018;48:356-361.
- 29. Healy LA, Ryan A, Doyle SL, Ní Bhuachalla ÉB, Cushen S, et al. Does prolonged enteral feeding with supplemental omega-3 fatty acids impact on recovery post-esophagectomy: results of a randomized double-blind trial. Ann Surg 2017;266:720-728.
- 30. Ida S, Hiki N, Cho H, Sakamaki K, Ito S, et al. Randomized clinical trial comparing standard diet with perioperative oral immunonutrition in total gastrectomy for gastric cancer. Br J Surg 2017;104:377-383.
- 31. Jantharapattana K, Orapipatpong O. Efficacy of EPA-enriched supplement compared with standard formula on body weight changes in malnourished patients with head and neck cancer undergone surgery: a randomized study. Head Neck 2020;42:188-197.
- 32. Kanat O, Cubukcu E, Avci N, Budak F, Ercan I, et al. Comparison of three different treatment modalities in the management of cancer cachexia. Tumori 2013;99:229-233.
- 33. Laviano A, Calder PC, Schols AM, Lonnqvist F, Bech M, et al. Safety and tolerability of targeted medical nutrition for cachexia in non-small-cell lung cancer: a randomized, double-blind, controlled pilot trial. Nutr Cancer 2020;72:439-450.
- 34. Mantovani G, Macciò A, Madeddu C, Serpe R, Massa E, et al. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist 2010;15:200-211.
- 35. Matsuda Y, Habu D, Lee S, Kishida S, Osugi H. Enteral diet enriched with ω-3 fatty acid improves oxygenation after thoracic esophagectomy for cancer: a randomized controlled trial. World J Surg 2017;41:1584-1594.
- 36. Mocellin MC, de Quadros Camargo C, de Souza Fabre ME, de Moraes Trindade EB. Fish oil effects on quality of life, body weight and free fat mass change in gastrointestinal cancer patients undergoing chemotherapy: a triple blind, randomized clinical trial. J Funct Foods 2017;31:113-122.
- 37. Mocellin MC, Pastore e Silva Jde A, Camargo Cde Q, Fabre ME, Gevaerd S, et al. Fish oil decreases C-reactive protein/albumin ratio improving nutritional prognosis and plasma fatty acid profile in colorectal cancer patients. Lipids 2013;48:879-888.
- 38. Moses AW, Slater C, Preston T, Barber MD, Fearon KC. Reduced total energy expenditure and physical activity in cachectic patients with pancreatic cancer can be modulated by an energy and protein dense oral supplement enriched with n-3 fatty acids. Br J Cancer 2004;90:996-1002.
- 39. Paixão EMDS, Oliveira ACM, Pizato N, Muniz-Junqueira MI, Magalhães KG, et al. The effects of EPA and DHA enriched fish oil on nutritional and immunological markers of treatment naïve breast cancer patients: a randomized double-blind controlled trial. Nutr J 2017;16:71.
- 40. Roca-Rodríguez MM, García-Almeida JM, Lupiañez-Pérez Y, Rico JM, Toledo M, et al. Effect of a specific supplement enriched with n-3 polyunsaturated fatty acids on markers of inflammation, oxidative stress and metabolic status of ear, nose and throat cancer patients. Oncol Rep 2014;31:405-414.
- 41. Sánchez-Lara K, Turcott JG, Juárez-Hernández E, Nuñez-Valencia C, Villanueva G, et al. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin Nutr 2014;33:1017-1023.
- 42. Silva Jde A, Trindade EB, Fabre ME, Menegotto VM, Gevaerd S, et al. Fish oil supplement alters markers of inflammatory and nutritional status in colorectal cancer patients. Nutr Cancer 2012;64:267-273.
- 43. Solís-Martínez O, Plasa-Carvalho V, Phillips-Sixtos G, Trujillo-Cabrera Y, Hernández-Cuellar A, et al. Effect of eicosapentaenoic acid on body composition and inflammation markers in patients with head and neck squamous cell cancer from a public hospital in Mexico. Nutr Cancer 2018;70:663-670.
- 44. Suzumura DN, Schleder JC, Appel MH, Naliwaiko K, Tanhoffer R, et al. Fish oil supplementation enhances pulmonary strength and endurance in women undergoing chemotherapy. Nutr Cancer 2016;68:935-942.
- 45. Wigmore SJ, Ross JA, Falconer JS, Plester CE, Tisdale MJ, et al. The effect of polyunsaturated fatty acids on the progress of cachexia in patients with pancreatic cancer. Nutrition 1996;12:S27-S30.
- 46. Gui L, Cheng M, Zheng M, Ning C, Huo Q. Effects of omega-3 fatty acid supplementation on nutritional status and inflammatory response in patients with stage II-III NSCLC undergoing postoperative chemotherapy: a double-blind randomized controlled trial. Front Nutr 2023;10:1266584.
- 47. Lam CN, Watt AE, Isenring EA, de van der Schueren MA, van der Meij BS. The effect of oral omega-3 polyunsaturated fatty acid supplementation on muscle maintenance and quality of life in patients with cancer: a systematic review and meta-analysis. Clin Nutr 2021;40:3815-3826.
- 48. Delpino FM, Figueiredo LM. Effects of omega-3 supplementation on lean body mass in cancer patients: a systematic review and meta-analysis. Eur J Clin Nutr 2022;76:1636-1645.
- 49. Ma YJ, Yu J, Xiao J, Cao BW. The consumption of omega-3 polyunsaturated fatty acids improves clinical outcomes and prognosis in pancreatic cancer patients: a systematic evaluation. Nutr Cancer 2015;67:112-118.
 , Sheida Zeraattalab-Motlagh3
, Sheida Zeraattalab-Motlagh3 , Reza Amiri Khosroshahi4
, Reza Amiri Khosroshahi4 , Amirhossein Hemmati4
, Amirhossein Hemmati4 , Morvarid Noormohammadi1,2
, Morvarid Noormohammadi1,2 , Hamed Mohammadi4
, Hamed Mohammadi4