ABSTRACT
Numerous studies have indicated that low levels of serum adiponectin are linked with the development of various chronic diseases. While some recent research has suggested that soy has a positive impact on serum adiponectin levels, the results are inconsistent. Therefore, we aim to conduct a thorough systematic review and meta-analysis of randomized controlled trials (RCTs) that investigate the effects of soy on serum adiponectin levels in adults. The search was conducted until March 2024 on PubMed, Scopus, Web of Science, and Cochrane Library databases to identify RCTs that studied the effects of soy supplementation on serum adiponectin levels. A random-effects model was used to pool the weighted mean differences (WMDs). Ten and nine RCTs were selected for the systematic review and meta-analysis, respectively. After analyzing data from 9 eligible RCTs, it was found that soy supplementation did not significantly impact the concentrations of adiponectin (WMD = −0.24 μg/mL; 95% confidence interval, −1.56 to 1.09; p = 0.72). However, there was significant heterogeneity between the studies (I2 = 89.8%, p < 0.001). Sensitivity analysis showed that overall estimates were not affected by the elimination of any study. We did not observe any evidence regarding publication bias. In conclusion, soy supplementation did not have a significant effect on adiponectin levels in adults. However, further RCTs are needed with longer intervention duration, higher doses, and studies conducted in different countries.
-
Keywords: Soy foods; Adiponectin; Soy protein; Systematic review; Meta-analysis
INTRODUCTION
Adiponectin is a pleiotropic hormone exclusively secreted by adipocytes and has important anti-inflammatory, anti-atherosclerotic, and anti-obesity effects [
1,
2]. It is also an insulin-sensitizing hormone, which plays a pivotal role in the regulation of energy homeostasis, inflammation, and cell proliferation [
2,
3]. Previous investigations have suggested that the low serum adiponectin concentration was associated with the incidence of various chronic diseases such as cardiovascular diseases (CVDs), diabetes, cancers, and chronic kidney disease [
3,
4,
5]. Adiponectin levels in humans can be increased through indirect methods such as weight loss or physical activity [
6,
7]. Therefore, improving the level of adiponectin is one of the important goals of health systems. Several studies have investigated the impact of pharmacological interventions and surgery on adiponectin levels [
8,
9]. In recent years, the effect of various dietary factors on improving the level of this hormone has been investigated [
10,
11,
12]. One such dietary component is soy foods.
Soy foods are commonly consumed in Asian diets but are less frequently consumed in Western diets [
13,
14]. Soy foods are rich in soluble fibers, plant protein, polyunsaturated fat, isoflavones, vitamins, and minerals combined with a low glycemic index [
15,
16]. Previous studies have found that consuming soy protein or isoflavones is linked to a lower risk of CVD risk factors, including hypertension, inflammation, obesity, blood lipid profile, glycemic control, and endothelial function [
17,
18,
19,
20]. Recently, lots of clinical trials have investigated the effects of soy and soy products on serum levels of adipokines, and have reported mixed findings [
21,
22,
23,
24,
25,
26,
27,
28,
29,
30]. The variability in study results may be due to differences in design, individual characteristics, soy dose, and supplemental duration. In addition, various meta-analyses in this field have produced differing results [
31,
32,
33,
34]. Therefore, we conducted a meta-analysis of randomized controlled trials (RCTs) to quantify the effects of soy on serum adiponectin levels in adults.
MATERIALS AND METHODS
The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [
35]. This review protocol was not published.
PubMed, Scopus, Web of Science, and Cochrane Library databases were systematically searched for articles published in English up to 30 March 2024, with no start-date restriction. In brief, search terms included: (soy OR tofu OR soybeans OR soymilk OR genistein OR daidzein OR isoflavone OR phytoestrogens) AND (adipokines OR adipocytokines OR adiponectin) AND (intervention OR “intervention study” OR “controlled trial” OR randomised OR randomised OR random OR randomly OR placebo OR assignment OR “clinical trial” OR trial OR assignment OR “randomised controlled trial” OR “randomised clinical trial” OR RCT OR blinded OR “double-blind” OR “double-blinded” OR trial OR “clinical trial” OR trials OR “Cross-Over Studies” OR “Cross-Over” OR “Cross-Over Study” OR parallel OR “parallel study” OR “parallel trial”). In addition, we manually checked all reference lists of the included articles and related reviews to ensure no relevant studies were overlooked.
Including and excluding criteria
Two investigators (J.K. and P.Sh.) independently reviewed all potentially relevant articles, and disagreement was resolved by discussion. The inclusion criteria of the study were as follows: 1) RCTs that were conducted on adults (participants ≥ 18 years old), and 2) RCTs that provided sufficient data on the baseline and final measures of adiponectin in both soy and control groups. The exclusion criteria of the study were as follows: 1) RCTs with an intervention duration of less than 2 weeks, 2) studies that investigated the effect of soy in combination with other plants, 3) studies that were not RCTs, and 3) RCTs that did not provide sufficient information regarding the outcome measures in the soy or control groups. If study populations were reported more than once, we used the result with a longer follow-up time.
Data extraction and quality assessment
The following information was extricated from each article: name of first author; year of publication; study design, location of study, total sample size, study duration, mean age, body mass index (BMI) and sex of participants, type of intervention and control, health status of the participants, and mean and standard deviation (SD) of outcome measures at the baseline and the final stage of the study. Two investigators (J.K. and P.Sh.) individually extracted the data using a standard extraction form. In cases where required data was not available in the published article or could not be extracted from figures, the corresponding author was contacted. The Cochrane Collaboration’s tool [
36], assesses the quality of RCTs based on random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias, was used by two authors for evaluating the study quality independently. For each criterion, the studies were either judged as meeting (low risk of bias), unclear risk of bias, or not meeting (high risk of bias) the criteria based on predetermined guidelines. Disagreements regarding data extraction and quality assessment were settled as described above.
The mean change value for adiponectin with their SDs was extracted from individual studies to calculate the weighted mean difference (WMD) and its standard errors between the soy and control groups, to be used as the effect size for favorable outcomes. The effect size was pooled using a random-effects model in conjunction with the DerSimonian and Laird method [
37]. Possible heterogeneity between studies and the percentage heterogeneity was calculated by using I
2 test, with values of 25%, 50%, and 75% regarded as a low, moderate, and high degree of heterogeneity, respectively [
38]. Subgroup analyses were conducted to investigate possible sources of heterogeneity based on intervention duration, study design, and baseline BMI. To evaluate whether the overall effect was steady, sensitivity analysis was performed by deleting one trail at a time, and the effect size was re-calculated. Publication bias was assessed using Egger’s [
39] regression test. All statistical analyses were conducted using STATA, version 11.2 (Stata Corp, College Station, TX, USA). The p values less than 0.05 were considered statistically significant.
RESULTS
Search results
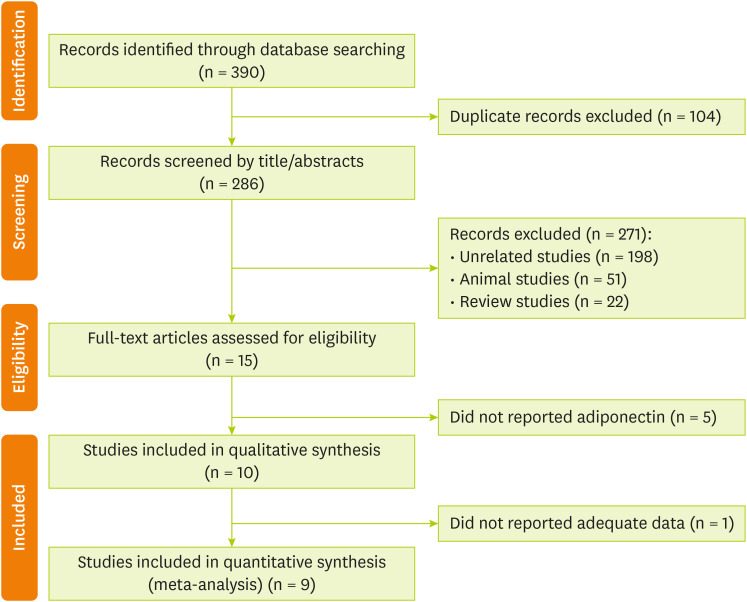
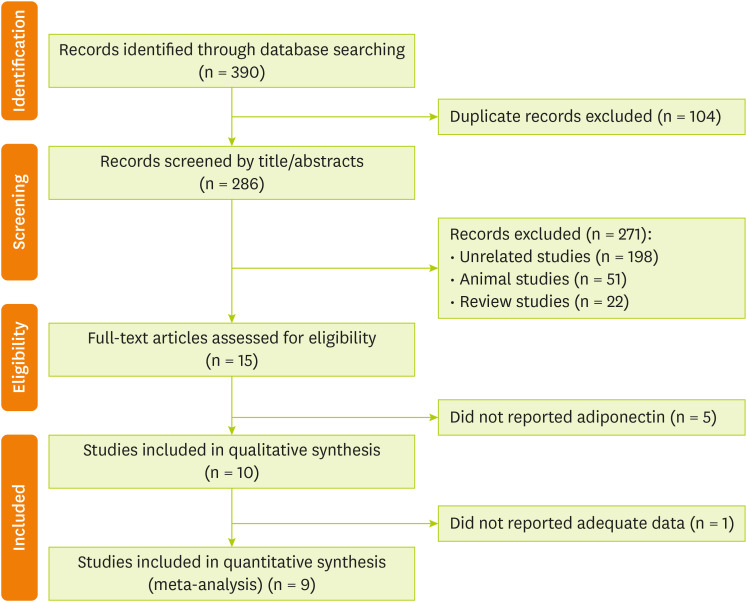
The search strategy retrieved 390 citations. After the removal of 104 duplicates, titles and abstracts of 286 references were screened. At this stage, 15 full-text articles were judged to be potentially relevant, of which 10 articles [
21,
22,
23,
24,
25,
26,
27,
28,
29,
30] satisfied the review eligibility criteria. The study selection flow diagram is presented in
Figure 1.
Figure 1Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow chart of the study selection process.
Characteristics of included studies
Included RCTs had small sample sizes (n = 25–183) and were conducted in the USA, Iran, England, Canada, Spain, and Brazil. Except for three studies, the remaining studies had a parallel design. The intervention period range was 4-96 weeks. The mean age of the included participants was 53 years. Six studies were conducted in post-menopausal women, one in subjects with metabolic syndrome, one in subjects with prostate cancer, one in hypertensive individuals, and one in subjects with rheumatoid arthritis. Detailed information on the included studies is summarized in
Table 1. The details related to the quality assessment of the studies using the Cochrane collaborations tool are reported in
Table 2.
Table 1Characteristics of the studies included in the meta-analysis
Table 1
|
Study (publication year, location) |
RCT design |
Sex, mean age (yr) |
Mean BMI |
Total sample size |
Duration (wk) |
Participants |
Intervention |
Control |
|
Maskarinec et al. [25] (2009, USA) |
Parallel |
Female (43) |
26 |
183 |
96 |
Post-menopausal women |
Soy products |
Regular diet |
|
Charles et al. [22] (2009, USA) |
Parallel |
Female (56) |
26 |
75 |
12 |
Post-menopausal women |
soy protein powder |
Placebo powder |
|
Christie et al. [21] (2010, England) |
Parallel |
Female (54) |
35 |
33 |
12 |
Obese post-menopausal women |
Soy shakes |
Casein without isoflavones |
|
Napora et al. [24] (2011, USA) |
Parallel |
Male (69) |
28 |
33 |
12 |
Prostate cancer |
Soy protein |
Whole milk protein |
|
Riesco et al. [28] (2012, Canada) |
Parallel |
Female (58) |
28 |
55 |
24 |
Post-menopausal women |
Exercise + soy extract |
Placebo + exercise |
|
Llaneza et al. [26] (2011, Spain) |
Parallel |
Female (56) |
34 |
87 |
24 |
Post-menopausal women |
Diet + exercise + soy isoflavones extract |
Diet + exercise |
|
Lozovoy et al. [27] (2012, Brazil) |
Parallel |
Female (47) |
35 |
30 |
12 |
Metabolic syndrome |
Soybean |
Usual diet |
|
Rebholz et al. [30] (2013, USA) |
Crossover |
Both (51) |
29 |
48 |
8 |
Hypertensive individuals |
Soybean protein |
Milk protein |
|
Nadadur et al. [23] (2016, USA) |
Parallel |
Female (58) |
28 |
37 |
8 |
Post-menopausal women |
Soy protein |
Control diet |
|
Mohammad-Shahi et al. [29] (2016, Iran) |
Crossover |
Female (45) |
29 |
25 |
4 |
Rheumatoid arthritis |
Soy milk |
Dairy milk |
Table 2Quality assessment of included studies based on Cochrane guidelines
Table 2
|
Study |
Random sequence generation |
Allocation concealment |
Blinding of participants, personnel |
Blinding of outcome assessment |
Incomplete outcome data |
Selective outcome reporting |
Other sources of bias |
Overall quality |
|
Maskarinec et al. [25] |
U |
U |
U |
L |
L |
U |
U |
Fair |
|
Charles et al. [22] |
L |
L |
L |
U |
L |
L |
U |
Good |
|
Christie et al. [21] |
L |
L |
L |
U |
L |
H |
L |
Good |
|
Napora et al. [24] |
L |
U |
L |
U |
L |
U |
U |
Good |
|
Riesco et al. [28] |
U |
L |
L |
U |
U |
U |
U |
Fair |
|
Llaneza et al. [26] |
L |
H |
L |
L |
L |
L |
U |
Good |
|
Lozovoy et al. [27] |
U |
U |
H |
U |
L |
L |
U |
Fair |
|
Rebholz et al. [30] |
L |
U |
L |
L |
U |
L |
U |
Good |
|
Nadadur et al. [23] |
L |
U |
L |
L |
U |
L |
U |
Good |
|
Mohammad-Shahi et al. [29] |
U |
U |
H |
H |
L |
U |
L |
Fair |
Findings of the systematic review
One of the included studies [
21] did not report enough data for analysis, so this study is only reported as a systematic review. This study found no significant change in adiponectin levels after three months of soy supplementation in women with hypertension compared to the control group.
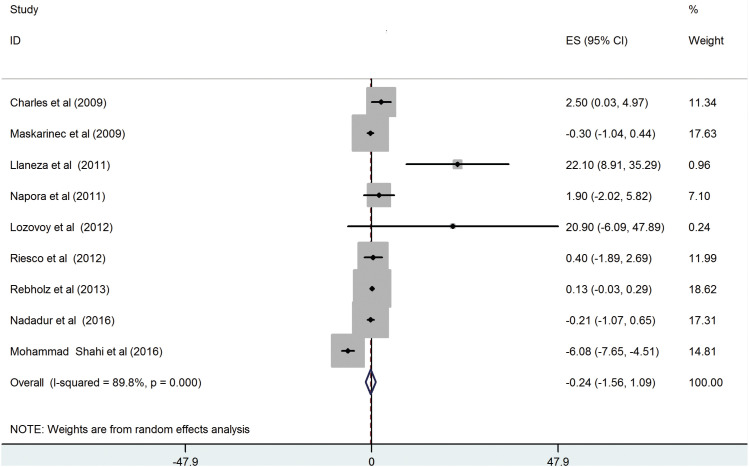
Nine RCTs [
22,
23,
24,
25,
26,
27,
28,
29,
30] were carried out to investigate the impact of soy and soy products on the serum concentration of adiponectin. These studies involved a total of 573 participants. The combined results of these studies showed that there was no significant change in the levels of serum adiponectin in the intervention group as compared to the control group (WMD = −0.24 μg/mL; 95% confidence interal, −1.56 to 1.09; p = 0.72). However, there was significant heterogeneity between the studies (I
2 = 89.8%, p < 0.001) (
Figure 2), and this could not be eliminated by conducting subgroup analyses (
Table 3). In addition, sensitivity analysis showed that any one specific study did not substantially influence the overall results. No evidence of publication bias was shown for the meta-analysis of the adiponectin (Egger’s test, p = 0.95).
Figure 2
Effect of soy supplementation on serum adiponectin levels.
ES, effect size; CI, confidence interval.
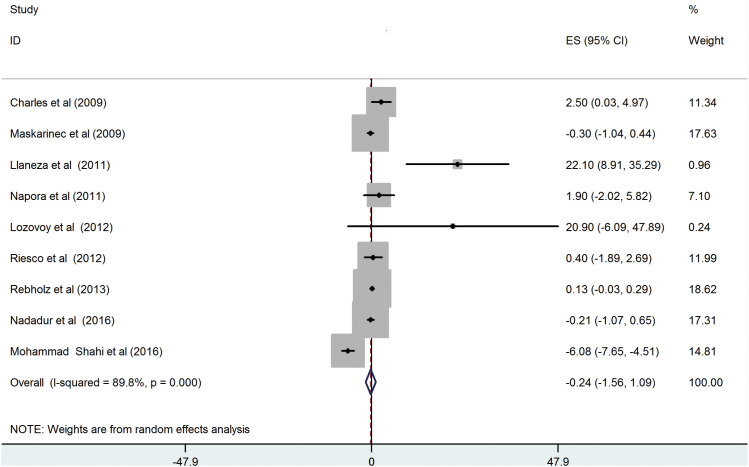
Table 3Subgroup analyses to assess the effect of soy supplementation on adiponectin levels
Table 3
|
Sub-grouped by |
No. of trials |
Effect size*
|
95% CI |
I2 (%) |
p for heterogeneity |
p for between subgroup heterogeneity |
|
Baseline BMI (kg/m2) |
|
|
|
|
|
< 0.001 |
|
< 30 |
7 |
0.08 |
−0.28 to 0.44 |
19.5 |
0.28 |
|
≥ 30 |
2 |
21.87 |
10.02 to 33.72 |
0.0 |
0.93 |
|
Duration (wk) |
|
|
|
|
|
0.90 |
|
≤ 8 |
3 |
−1.90 |
−4.36 to 0.56 |
96.7 |
< 0.001 |
|
> 8 |
6 |
1.75 |
−0.65 to 4.16 |
73.1 |
0.002 |
|
RCT design |
|
|
|
|
|
0.76 |
|
Parallel |
7 |
0.77 |
−0.61 to 2.15 |
68.2 |
0.004 |
|
Crossover |
2 |
−2.92 |
−9.01 to 3.16 |
98.3 |
< 0.001 |
DISCUSSION
In the present meta-analysis, we aimed to evaluate the effects of soy supplementation on adiponectin levels in adults. Data analysis showed that soy supplementation had no significant effect on adiponectin. Results of subgroup analysis revealed that subgroup analysis based on trial design, and intervention duration could not change their results. However, when sub-grouped by BMI, the results were significant in obese participants. Due to the limited number of studies in the subsets, interpretation of these results should be done with caution.
The results of the present study are remarkably similar to previous meta-analyses [
31,
32,
34]. The results of these studies indicate that the consumption of soy and its compounds does not have a significant effect on the level of serum adiponectin. However, the results of this study contradict those of a previous meta-analysis [
33], which found that soy isoflavones increase adiponectin levels in the postmenopausal women. The reason for the difference observed could be attributed to the various factors. The onset of the postmenopausal period is associated with increased abdominal fat and central obesity, which can lead to metabolic dysfunctions such as insulin resistance, and dyslipidemia, especially when accompanied by visceral fat accumulation. As a result, these individuals have lower levels of adiponectin, making them more susceptible to interventions aimed at increasing their levels [
33,
40,
41].
Although, based on the present results, soy consumption does not have a significant effect on adiponectin levels, some mechanisms have been suggested for the effect of soy on increasing adiponectin levels. Previous studies have shown that soy may reduce obesity markers and thereby increase adiponectin levels [
42,
43]. It appears that the high sensitivity of adipose tissue to oxidative stress and inflammation leads to a decrease in the gene expression of adiponectin in 3T3-L1 adipocytes when exposed to elevated levels of oxidative stress agents [
1,
31,
44]. Therefore, antioxidant factors such as soy may enhance the secretion of adiponectin from adipose tissue [
45]. There is another mechanism that may be linked to the increase in adiponectin, which is the nitric oxide (NO) pathway. Previous studies have found that soy and its products can boost the production of NO in endothelial cells, leading to an increased secretion of adiponectin [
31].
Although relatively common side effects were not discussed in any RCT analysis, we cannot neglect allergic reactions caused by soy supplementation. The most common side effects reported after soy intake are digestive upsets, such as constipation and diarrhea. In addition, soy may alter thyroid function in people who are deficient in iodine [
46,
47,
48].
Like all reviews, there are some potential limitations in the present study. First, the number of eligible studies in this meta-analysis was relatively small, which may have biased the results to some extent. Second, the high heterogeneity among studies may reduce the validity of the results. Third, in most studies, adiponectin has been reported as a secondary factor and not a primary objective. Fourth, due to the limited number of studies, we were unable to present results for the gender-separated subgroup, which could be a significant factor. In addition, most of the included studies have been done in the USA, which makes it difficult to generalize the results to the rest of the world. None of the included studies measured serum polyphenol content, making it difficult to assess participant compliance. Finally, most studies failed to control for confounders such as diet and physical activity levels, which could impact the results.
In conclusion, we found that soy may not increase adiponectin levels. However, more RCTs with longer intervention duration, higher doses, and studies in different countries are still needed. Furthermore, the confounding effects of diet and physical activity should be adjusted.
NOTES
-
Conflict of Interest: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Karimian J, Shekarchizadeh-Esfahani P.
Data curation: Karimian J, Shekarchizadeh-Esfahani P.
Formal analysis: Karimian J.
Funding acquisition: Karimian J.
Investigation: Karimian J, Shekarchizadeh-Esfahani P.
Methodology: Karimian J, Shekarchizadeh-Esfahani P.
Project administration: Karimian J, Shekarchizadeh-Esfahani P.
Resources: Karimian J, Shekarchizadeh-Esfahani P.
Software: Karimian J.
Supervision: Shekarchizadeh-Esfahani P.
Validation: Karimian J, Shekarchizadeh-Esfahani P.
Visualization: Karimian J, Shekarchizadeh-Esfahani P.
Writing - original draft: Karimian J, Shekarchizadeh-Esfahani P.
Writing - review & editing: Karimian J, Shekarchizadeh-Esfahani P.
REFERENCES
- 1. Esmaili S, Hemmati M, Karamian M. Physiological role of adiponectin in different tissues: a review. Arch Physiol Biochem 2020;126:67-73.
- 2. Oh DK, Ciaraldi T, Henry RR. Adiponectin in health and disease. Diabetes Obes Metab 2007;9:282-289.
- 3. Kawano J, Arora R. The role of adiponectin in obesity, diabetes, and cardiovascular disease. J Cardiometab Syndr 2009;4:44-49.
- 4. Choi HM, Doss HM, Kim KS. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int J Mol Sci 2020;21:1219.
- 5. Przybyciński J, Dziedziejko V, Puchałowicz K, Domański L, Pawlik A. Adiponectin in chronic kidney disease. Int J Mol Sci 2020;21:9375.
- 6. Simpson KA, Singh MA. Effects of exercise on adiponectin: a systematic review. Obesity (Silver Spring) 2008;16:241-256.
- 7. Guerre-Millo M. Adiponectin: an update. Diabetes Metab 2008;34:12-18.
- 8. Li FY, Lam KS, Xu A. YL Li F. Therapeutic perspectives for adiponectin: an update. Curr Med Chem 2012;19:5513-5523.
- 9. Askarpour M, Alizadeh S, Hadi A, Symonds ME, Miraghajani M, Sheikhi A, Ghaedi E. Effect of bariatric surgery on the circulating level of adiponectin, chemerin, plasminogen activator inhibitor-1, leptin, resistin, and visfatin: a systematic review and meta-analysis. Horm Metab Res 2020;52:207-215.
- 10. Janiszewska J, Ostrowska J, Szostak-Węgierek D. The influence of nutrition on adiponectin: a narrative review. Nutrients 2021;13:1394.
- 11. Silva FM, de Almeida JC, Feoli AM. Effect of diet on adiponectin levels in blood. Nutr Rev 2011;69:599-612.
- 12. Clark CC, Ghaedi E, Arab A, Pourmasoumi M, Hadi A. The effect of curcumin supplementation on circulating adiponectin: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Syndr 2019;13:2819-2825.
- 13. D’Adamo CR, Sahin A. Soy foods and supplementation: a review of commonly perceived health benefits and risks. Altern Ther Health Med 2014;20(Suppl 1):39-51.
- 14. Rizzo G, Baroni L. Soy, soy foods and their role in vegetarian diets. Nutrients 2018;10:43.
- 15. Lokuruka M. Soybean nutritional properties: the good and the bad about soy foods consumption: a review. Afr J Food Agric Nutr Dev 2010;10:2439-2459.
- 16. Tang J, Wan Y, Zhao M, Zhong H, Zheng JS, Feng F. Legume and soy intake and risk of type 2 diabetes: a systematic review and meta-analysis of prospective cohort studies. Am J Clin Nutr 2020;111:677-688.
- 17. Mosallanezhad Z, Mahmoodi M, Ranjbar S, Hosseini R, Clark CC, Carson-Chahhoud K, Norouzi Z, Abbasian A, Sohrabi Z, Jalali M. Soy intake is associated with lowering blood pressure in adults: a systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Complement Ther Med 2021;59:102692.
- 18. Luvián-Morales J, Varela-Castillo FO, Flores-Cisneros L, Cetina-Pérez L, Castro-Eguiluz D. Functional foods modulating inflammation and metabolism in chronic diseases: a systematic review. Crit Rev Food Sci Nutr 2022;62:4371-4392.
- 19. Li N, Wu X, Zhuang W, Xia L, Chen Y, Zhao R, Yi M, Wan Q, Du L, Zhou Y. Soy and isoflavone consumption and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomized trials in humans. Mol Nutr Food Res 2020;64:e1900751.
- 20. Man B, Cui C, Zhang X, Sugiyama D, Barinas-Mitchell E, Sekikawa A. The effect of soy isoflavones on arterial stiffness: a systematic review and meta-analysis of randomized controlled trials. Eur J Nutr 2021;60:603-614.
- 21. Christie DR, Grant J, Darnell BE, Chapman VR, Gastaldelli A, Sites CK. Metabolic effects of soy supplementation in postmenopausal Caucasian and African American women: a randomized, placebo-controlled trial. Am J Obstet Gynecol 2010;203:153.e1-153.e9.
- 22. Charles C, Yuskavage J, Carlson O, John M, Tagalicud AS, Maggio M, Muller DC, Egan J, Basaria S. Effects of high-dose isoflavones on metabolic and inflammatory markers in healthy postmenopausal women. Menopause 2009;16:395-400.
- 23. Nadadur M, Stanczyk FZ, Tseng CC, Kim L, Wu AH. The effect of reduced dietary fat and soy supplementation on circulating adipocytokines in postmenopausal women: a randomized controlled 2-month trial. Nutr Cancer 2016;68:554-559.
- 24. Napora JK, Short RG, Muller DC, Carlson OD, Odetunde JO, Xu X, Carducci M, Travison TG, Maggio M, Egan JM, Basaria S. High-dose isoflavones do not improve metabolic and inflammatory parameters in androgen-deprived men with prostate cancer. J Androl 2011;32:40-48.
- 25. Maskarinec G, Steude JS, Franke AA, Cooney RV. Inflammatory markers in a 2-year soy intervention among premenopausal women. J Inflamm (Lond) 2009;6:9.
- 26. Llaneza P, González C, Fernandez-Iñarrea J, Alonso A, Diaz F, Arnott I, Ferrer-Barriendos J. Soy isoflavones, diet and physical exercise modify serum cytokines in healthy obese postmenopausal women. Phytomedicine 2011;18:245-250.
- 27. Simão AN, Lozovoy MA, Bahls LD, Morimoto HK, Simão TN, Matsuo T, Dichi I. Blood pressure decrease with ingestion of a soya product (kinako) or fish oil in women with the metabolic syndrome: role of adiponectin and nitric oxide. Br J Nutr 2012;108:1435-1442.
- 28. Riesco E, Choquette S, Audet M, Lebon J, Tessier D, Dionne IJ. Effect of exercise training combined with phytoestrogens on adipokines and C-reactive protein in postmenopausal women: a randomized trial. Metabolism 2012;61:273-280.
- 29. Mohammad-Shahi M, Mowla K, Haidari F, Zarei M, Choghakhori R. Soy milk consumption, markers of inflammation and oxidative stress in women with rheumatoid arthritis: a randomised cross-over clinical trial. Nutr Diet 2016;73:139-145.
- 30. Rebholz CM, Reynolds K, Wofford MR, Chen J, Kelly TN, Mei H, Whelton PK, He J. Effect of soybean protein on novel cardiovascular disease risk factors: a randomized controlled trial. Eur J Clin Nutr 2013;67:58-63.
- 31. Mahmudiono T, Khaydarov NK, Jasim SA, Hammid AT, Failoc-Rojas VE, Shalaby MN, Jannat B, Nouri M, Fadel A. Systematic review and meta-analysis of randomized, controlled trials on the effects of soy and soy products supplementation on serum adiponectin levels. Diabetes Metab Syndr 2022;16:102558.
- 32. Hariri M, Amirkalali B, Mollanoroozy E, Gholami A. Can soy isoflavones in combination with soy protein change serum concentration of adiponectin and resistin? A systematic review and meta-analysis on randomized clinical trials. Food Sci Nutr 2022;10:4126-4138.
- 33. Tutunchi S, Koushki M, Amiri-Dashatan N, Khodabandehloo H, Hosseini H, Panahi G, Hashemi J, Karbalaee-Hasani A, Majidi Z, Rezaei-Tavirani M. Effect of soy isoflavones supplementation on adiponectin levels in postmenopausal women: a meta-analysis. J Pharm Nutr Sci 2021;11:184-195.
- 34. Moosavian SP, Rahimlou M, Asbaghi O, Moradi S, Marx W, Paknahad Z. The effect of soy products on circulating adiponectin and leptin concentration in adults: a systematic review and meta-analysis of randomised controlled trials. Int J Clin Pract 2021;75:e14100.
- 35. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097.
- 36. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA. Cochrane Bias Methods Group. Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928.
- 37. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007;28:105-114.
- 38. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-560.
- 39. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-634.
- 40. Engin A. Adiponectin-resistance in obesity. In Engin A, Engin A, eds, ddObesity and lipotoxicity. Cham: Springer; 2017, pp 415-441.
- 41. Ricci R, Bevilacqua F. The potential role of leptin and adiponectin in obesity: a comparative review. Vet J 2012;191:292-298.
- 42. Akhlaghi M, Zare M, Nouripour F. Effect of soy and soy isoflavones on obesity-related anthropometric measures: a systematic review and meta-analysis of randomized controlled clinical trials. Adv Nutr 2017;8:705-717.
- 43. Mu Y, Kou T, Wei B, Lu X, Liu J, Tian H, Zhang W, Liu B, Li H, Cui W, Wang Q. Soy products ameliorate obesity-related anthropometric indicators in overweight or obese Asian and non-menopausal women: a meta-analysis of randomized controlled trials. Nutrients 2019;11:2790.
- 44. Khoramipour K, Chamari K, Hekmatikar AA, Ziyaiyan A, Taherkhani S, Elguindy NM, Bragazzi NL. Adiponectin: Structure, physiological functions, role in diseases, and effects of nutrition. Nutrients 2021;13:1180.
- 45. Morvaridzadeh M, Nachvak SM, Agah S, Sepidarkish M, Dehghani F, Rahimlou M, Pizarro AB, Heshmati J. Effect of soy products and isoflavones on oxidative stress parameters: a systematic review and meta-analysis of randomized controlled trials. Food Res Int 2020;137:109578.
- 46. Sukalingam K, Ganesan K, Das S, Thent ZC. An insight into the harmful effects of soy protein: a review. Clin Ter 2015;166:131-139.
- 47. Jargin SV. Soy and phytoestrogens: possible side effects. Ger Med Sci 2014;12:Doc18.
- 48. Munro IC, Harwood M, Hlywka JJ, Stephen AM, Doull J, Flamm WG, Adlercreutz H. Soy isoflavones: a safety review. Nutr Rev 2003;61:1-33.