ABSTRACT
Estimating the nutritional requirements for pediatric patients requires a comprehensive approach with various factors including age, gender, body mass index, and physical activity level, due to the significant growth and developmental changes observed in this population. This complexity renders the use of a simplistic generalization or a standard formula impractical. A number of methodologies have been established to calculate nutritional needs for the pediatric population. However, the application of these methodologies is challenging due to the variability in the aforementioned factors. Determining nutritional requirements for pediatric patients with underlying medical conditions is complicate, influenced by variables such as the nature of the illness, treatment modalities, and the patient’s overall condition. Nutritional support in severely traumatically brain-injured pediatric patients is directly correlated with prognosis and growth outcomes. Therefore, this case study aims to validate existing methodologies for estimating nutritional requirements in pediatric patients with severe traumatic brain injury and to provide primary data for the development of effective nutritional support strategies. A case of a 5-year-old male patient admitted to the intensive care unit due to severe traumatic brain injury is examined. Future case studies and ongoing research are imperative to ensure the safe and effective nutritional support of pediatric patients with severe traumatic brain injury.
-
Keywords: Nutritional support; Enteral nutrition; Pediatrics; Traumatic brain injury
INTRODUCTION
Nutritional support is critical in the therapeutic management of pediatric patients with severe traumatic injuries [
1]. Accurate estimation of nutritional requirements is fundamental to this support [
2]. Unlike adult patients, estimating nutritional needs in pediatric patients is complex due to their rapid growth and development, requiring consideration of factors such as age, gender, body mass index (BMI), and physical activity level [
3,
4]. Consequently, applying a simplistic generalization or common formula is challenging [
5]. Therefore, unless specific nutritional considerations for disease treatment are necessary, the Korean Dietary Reference Intakes (KDRIs) for children of the corresponding age are typically followed [
6].
In instances where nutritional status is compromised or nutritional considerations due to illness are required, factors such as the patient’s age, gender, growth status, disease state, and nutritional status are evaluated to determine nutritional needs [
7,
8].
Various methodologies have been developed to estimate the nutritional requirements of pediatric patients [
9]. Typically, energy requirements are calculated using methods based on basal metabolic rate (BMR), weight-based energy needs, and dietary reference standards [
10]. Commonly used equations for calculating BMR include the Harris-Benedict equation, WHO equation, and Schofield equation [
11,
12,
13]. Protein requirements are generally adjusted based on disease conditions using the KDRIs [
5]. However, uniformly applying these developed methods is challenging due to the heterogeneous nature of diseases, treatment modalities, and patient conditions [
4,
10].
Nutritional support in pediatric patients with severe traumatic brain injury (TBI) is directly associated with brain development, functional recovery, prevention of complications, and overall growth and development [
1,
3].
Therefore, this case report aims to validate existing methodologies for estimating nutritional requirements and provide foundational data for effective nutritional support strategies in pediatric patients with severe TBI through intensive nutritional support [
2,
5,
7].
This study was approved by the Institutional Review Board (IRB) of Kyung Hee University Hospital (IRB File No. KHUH 2024-03-013).
CASE
A 5-year-old male patient was admitted to the intensive care unit (ICU) with severe TBI. Upon admission, his height was 114 cm and his weight was 23 kg, corresponding to a percent ideal body weight of 112.7%. His height was at the 51.5th percentile, weight at the 80.4th percentile, and BMI was 17.7 (87.3rd percentile). His nutritional status was adequate; however, he presented in a stuporous state. The total length of stay (LOS) was 298 days, with a total of 33 consultations by the nutritional support team (NST).
ICU LOS #0–8, First NST consult
On the first day of ICU admission, the patient underwent a craniotomy for an acute right subdural hematoma. Six days later, due to right intracerebral hemorrhage and right cerebral infarction, a decompressive craniectomy was performed. The patient was receiving vasopressor therapy with norepinephrine and was maintained on mechanical ventilation. The patient was kept nil per os (NPO) due to surgical risk and was only provided with 5% dextrose at a rate of 40 cc/hr. On the 8th day, a NST consultation was requested to initiate enteral nutrition. Using the Harris-Benedict equation, the patient's energy requirements were estimated to be 1,195 kcal, and protein requirements were calculated based on the current body weight with a coefficient of 1.1–1.3, resulting in an estimated protein requirement of 25.3–29.9 g/day.
ICU LOS #9–16, 2–3rd NST consults
On the 11th day, enteral nutrition was initiated at a rate of 20 cc/hr, but was halted due to the need for additional surgery, maintaining NPO status with 5% dextrose at 40 cc/hr. On the 14th day, the patient underwent an epidural and subgaleal hematoma evacuation on the right side due to a subgaleal hematoma. A tracheostomy was performed on the 16th day. Due to the risk of further surgery, NPO status was maintained with 5% dextrose at 40 cc/hr, Primene 10% at 250 cc/day, and SMOF lipid 20% at 100 cc/day. There were no changes in the patient’s energy and protein requirements. From the 16th day, the energy intake was 680 kcal/day and protein intake was 25g/day. Mechanical ventilation and continuous vasopressor support were maintained. By the 15th day, the patient’s weight had decreased by 1 kg.
ICU LOS #17–28, 4th NST consults
On the 23rd day, the patient’s weight had decreased by 1.7 kg since admission. Starting on the 24th day, SmofKabiven was administered at 30 cc/hr along with 10% dextrose at 20 cc/hr. From this day, the patient’s energy intake was 965 kcal/day and protein intake was 36.6 g/day. On the 26th day, enteral nutrition was reintroduced at 100 cc four times a day (qid) at a rate of 50 cc/hr, and gradually increased to 240 cc qid (80 cc/hr). By this period, the final energy intake was 960 kcal/day and protein intake was 43.2 g/day.
ICU LOS #29–38, 5–6th NST consults
On the 29th day, a ventriculoperitoneal shunt was performed via the left Kocher’s point due to communicating hydrocephalus. On the 36th day, the patient underwent a cranioplasty with autologous bone and partial temporal lobectomy on the right side due to a skull defect. Following complications such as wound dehiscence, skull exposure, and scalp defect, reconstruction was performed using an occipital artery perforator-based bipedicled flap and ADM graft. Coverage was achieved with Matriderm and split-thickness skin graft (STSG) from the right posterior thigh and buttock, along with biological dressing at the STSG donor site with amnion.
Three days postoperatively, the patient experienced wound disruption and a postoperative hematoma, necessitating hematoma evacuation, closure, and local flap coverage. During this period, the patient was kept NPO due to the potential for additional surgeries, receiving SmofKabiven at 30 cc/hr and 10% dextrose at 20 cc/hr. The energy intake was maintained at 965 kcal/day with a protein intake of 36.6 g/day.
ICU LOS #39–56, 7–8th NST consults
On the 41st day, the patient underwent hematoma evacuation and wound closure, followed by a craniotomy and hematoma evacuation on the right side. Three days later, additional procedures were performed including hematoma evacuation, ADM graft placement, and wound closure, along with another craniotomy and hematoma evacuation on the right side. During this period, SmofKabiven was administered at 40 cc/hr. The patient’s energy intake was maintained at 1,040 kcal/day, with a protein intake of 48 g/day.
ICU LOS #57–77, 9–11th NST consults
The patient’s condition stabilized, and on the 57th day, enteral nutrition was initiated with fiber-free RTH at 50 cc/day (10 cc/hr) once daily. The volume was gradually increased, but due to intolerance, it was discontinued on the 61st day, and SmofKabiven was maintained at 40 cc/hr. On the 72nd day, fiber-free RTH was reintroduced at 50 cc QID at 25 cc/hr. By the 76th day, enteral nutrition was increased to 150 cc QID, and SmofKabiven was reduced to 30 cc/hr. The patient was transferred to a general ward on the 77th day, and their level of consciousness remained stuporous. During this period, the average energy intake was approximately 1,100 kcal/day, with a protein intake of about 50 g/day.
GW #1(LOS #78–92), 12–13th NST consults
After being transferred to the general ward, the patient’s nutrition was changed to a standard formula containing fiber. The volume was gradually increased, and by the 12th day, the rate of RTH was increased to 200 cc QID, at which point parenteral nutrition was discontinued. The enteral nutrition was maintained at 250 cc QID. During this period, the average energy intake was approximately 1,100 kcal/day, with a protein intake of about 45 g/day. A rehabilitation therapy was also initiated during the same period, although his mental status remained stuporous.
GW #15–47(LOS #93–122), 14–17th NST consults
On the 15th day in the general ward, the patient underwent debridement and aseptic dressing performed by the plastic surgery department. On the 22nd day, additional debridement and wiring were conducted. During this period, the patient was maintained on 250 cc QID (1,000 kcal/day, protein 43 g/day).
GW #48–158(LOS #123~232), 18–30th NST consults
At the request of the caregiver, the feeding schedule was changed on the 48th day to three times a day at 300 cc each time, and this regimen was maintained until the 71st day. On the 71st day, the plastic surgery department performed irrigation and debridement, bone shaving, wide dissection, and local transpositional flap coverage. Enteral nutrition was provided up to the 116th day at 300-350-300-350 cc daily, totaling 1,300 kcal/day with 55 g of protein. A VFSS (Video Fluoroscopic Swallowing Study) was conducted on the 117th day, but severe dysphagia prevented the initiation of assisted feeding. On the 128th day, considering weight gain, the enteral nutrition was reduced to 300 cc QID (1,200 kcal/day, 51g of protein).
GW #159–224(LOS #233~298), 31–33rd NST consults
Due to continuous weight gain, the patient was maintained on a diet providing 1,000 kcal/day and 43 g of protein from the 159th day onwards. On the 198th day, a VFSS was conducted, and the patient successfully consumed 100 cc of puree without aspiration. Subsequently, enteral nutrition and puree feeding were maintained until discharge
Changes in anthropometric data, active factor, injury factor, biochemical data, total energy intake, energy requirement, total protein intake, minimum and maximum protein requirement are presented in
Tables 1,
2,
Figures 1, and
2.
Table 1Changes in anthropometric information, active and injury factor of the study case
Table 1
|
Variables |
LOS (day)/NST consult |
|
9/1st |
31/5th |
59/9th |
80/12th |
108/16th |
131/19th |
162/23rd |
190/27th |
233/31st |
272/33rd |
|
Age (mon) |
68 |
69 |
70 |
71 |
72 |
73 |
74 |
75 |
76 |
77 |
|
Height (cm) |
114 |
114 |
114 |
114 |
114 |
115 |
117 |
122 |
124 |
124 |
|
Body weight (kg) |
23.0 |
23.0 |
22.8 |
23.1 |
24.3 |
23.8 |
26.5 |
27.3 |
28.2 |
28.6 |
|
50th percentile weight-for-height (kg) |
20.4 |
20.4 |
20.4 |
20.4 |
20.4 |
20.4 |
21.5 |
23.8 |
24.9 |
24.9 |
|
Weight-for-height percentage (%) |
112.7 |
112.7 |
111.8 |
113.2 |
119.1 |
116.7 |
123.3 |
114.7 |
113.3 |
114.9 |
|
Height-for-age percentile |
47.0 |
47.0 |
42.5 |
38.1 |
34.0 |
38.0 |
50.4 |
82.5 |
89.1 |
86.8 |
|
Weight-for-age percentile |
78.2 |
78.2 |
73.9 |
74.5 |
82.3 |
81.6 |
90.8 |
92.4 |
94.0 |
94.1 |
|
BMI-for-age percentile |
87.0 |
87.0 |
84.0 |
87.7 |
94.7 |
93.0 |
97.1 |
90.5 |
89.9 |
91.9 |
|
Activity Factor |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.1 |
1.1 |
1.1 |
|
Injury Stress Factor |
1.3 |
1.2 |
1.2 |
1.2 |
1.3 |
1.3 |
1.0 |
1.0 |
1.0 |
1.0 |
Table 2Changes inbiochemical data of the study case
Table 2
|
Variables |
LOS (day)/NST consult |
|
9/1st |
31/5th |
59/9th |
80/12th |
108/16th |
131/19th |
162/23rd |
190/27th |
233/31st |
272/33rd |
|
BUN (mg/dL) |
10 |
17 |
16 |
24 |
21 |
20 |
16 |
17 |
14 |
10 |
|
Creatinine (mg/dL) |
0.23 |
0.24 |
0.20 |
0.20 |
0.39 |
0.70 |
0.27 |
0.32 |
0.31 |
0.35 |
|
Na (mmol/L) |
142 |
137 |
132 |
135 |
133 |
138 |
139 |
136 |
136 |
138 |
|
K (mmol/L) |
3.2 |
3.9 |
4.1 |
5.0 |
5.0 |
4.7 |
4.0 |
4.0 |
4.2 |
4.0 |
|
Cl (mmol/L) |
110 |
106 |
101 |
103 |
102 |
107 |
103 |
103 |
103 |
104 |
|
CRP (mg/dL) |
5.06 |
1.13 |
1.87 |
0.54 |
0 |
0 |
0 |
0 |
0 |
0 |
|
Protein (g/dL) |
4.7 |
5.8 |
6.3 |
6.2 |
6.9 |
6.2 |
6.6 |
7.8 |
7.4 |
7.6 |
|
Albumin (g/dL) |
3 |
3.4 |
3.7 |
3.7 |
4 |
3.8 |
3.9 |
4.1 |
4 |
4.3 |
|
AST (U/L) |
49 |
48 |
29 |
600 |
31 |
24 |
22 |
21 |
17 |
17 |
|
ALT (U/L) |
55 |
30 |
18 |
267 |
42 |
39 |
27 |
17 |
10 |
11 |
|
Hemoglobin (g/dL) |
10.1 |
8.2 |
10.9 |
12.4 |
10.6 |
10.0 |
10.2 |
13.0 |
12.2 |
12.8 |
|
Hematocrit (%) |
27.9 |
24.4 |
31.8 |
36.3 |
29.6 |
29.3 |
30.2 |
39.8 |
37.5 |
3.9 |
|
TLC (cells/mm3) |
1,963 |
7,67 |
1,175 |
1,220 |
1,550 |
1,768 |
1,773 |
1,881 |
1,788 |
2,503 |
Figure 1
Total energy intake and requirement of the study case. The three energy requirements were determined taking both activity and injury factors into account.
SF, Schofield equation; WHO, WHO equation; HB, HB equation.
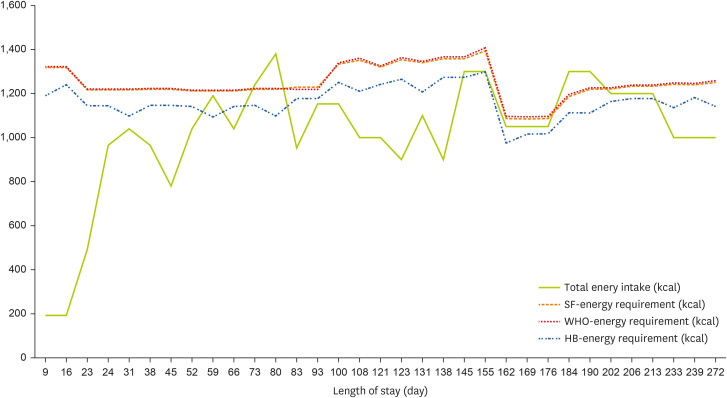
Figure 2Total protein intake, minimum and maximum protein requirement. The minimum protein requirement was determined by multiplying the actual body weight by a factor of 1.5, while the maximum protein requirement was determined by multiplying the actual body weight by a factor of 2.0.

DISCUSSION
This case report highlights the dynamic changes in physical parameters, nutritional status, nutritional requirements, and nutritional provision over an eight-month period in a pediatric patient with severe TBI. The complexity of managing nutritional support in such cases underscores the importance of individualized and continuous nutritional assessment and intervention.
Nutritional support is critical for the optimal recovery of children with severe TBI. Proper nutrition aids in managing the complex physiological changes that occur post-injury and supports overall recovery [
14]. Estimating energy requirements in pediatric patients typically involves using the Schofield equation or WHO equations to determine the BMR, which is then adjusted using activity and injury factors [
12,
13]. Protein requirements are generally set at 1.5 to 2.0 grams per kilogram of body weight [
5]. In cases of severe TBI in children, higher protein intake may be necessary to support wound healing and recovery processes.
In this case, the patient exhibited significant energy requirements initially due to severe TBI and multiple surgical interventions. During the recovery phase in the ICU and subsequent transfer to the general ward, the predicted caloric intake was not fully achieved. Despite this, the patient did not encounter any impediments in growth or weight gain. Remarkably, for the three months preceding discharge, the patient continued to gain weight even though the caloric intake provided was below the estimated BMR. This observation suggests that the patient’s metabolic demands were met effectively, possibly due to efficient utilization of the provided nutrition and the body’s adaptive mechanisms during the recovery process.
Pediatric brains generally consume a very high amount of energy. Research indicates that, particularly during early brain development, the brain can account for nearly half of the body's total energy expenditure [
15]. This significant energy demand during brain development is essential for supporting rapid neural growth and cognitive functions. In cases of brain injury, there is a reduction in the energy utilized by the brain, which may influence the actual BMR required. Additionally, during that period, there was no evidence of edema that could have influenced weight gain This reduction in energy needs could be a factor in the observed decrease in necessary energy intake while still maintaining growth and weight gain.
In pediatric patients with brain injuries, it is essential to regularly adjust nutritional requirements based on the extent of the brain injury and the patient's activity levels [
6]. Periodic assessments and consultations with a Nutrition Support Team are recommended to monitor the patient's growth and nutritional status. These evaluations help ensure that nutritional interventions are implemented at optimal times to improve the patient’s overall nutritional status.
Ongoing case reports and continuous research are necessary to develop safe and effective nutritional support strategies for pediatric patients with severe TBI. Such efforts will contribute to ensuring the safety and efficacy of nutritional interventions, thereby supporting the recovery and growth of these vulnerable patients.
NOTES
-
Conflict of Interest: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Lee IS, Kang K.
Data curation: Lee IS.
Investigation: Lee IS.
Writing - original draft: Lee IS.
Writing - review & editing: Lee IS, Kang K, Chung YM, Lee J.
REFERENCES
- 1. Elliott E, Shoykhet M, Bell MJ, Wai K. Nutritional support for pediatric severe traumatic brain injury. Front Pediatr 2022;10:904654.
- 2. Iyer R, Bansal A. What do we know about optimal nutritional strategies in children with pediatric acute respiratory distress syndrome? Ann Transl Med 2019;7:510.
- 3. Lucke-Wold BP, Logsdon AF, Nguyen L, Eltanahay A, Turner RC, et al. Supplements, nutrition, and alternative therapies for the treatment of traumatic brain injury. Nutr Neurosci 2018;21:79-91.
- 4. Larsen BM, Beggs MR, Leong AY, Kang SH, Persad R, et al. Can energy intake alter clinical and hospital outcomes in PICU? Clin Nutr ESPEN 2018;24:41-46.
- 5. Mehta NM, Skillman HE, Irving SY, Coss-Bu JA, Vermilyea S, et al. Guidelines for the provision and assessment of nutrition support therapy in the pediatric critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr 2017;41:706-742.
- 6. Mikhailov TA, Kuhn EM, Manzi J, Christensen M, Collins M, et al. Early enteral nutrition is associated with lower mortality in critically ill children. JPEN J Parenter Enteral Nutr 2014;38:459-466.
- 7. Mtaweh H, Smith R, Kochanek PM, Wisniewski SR, Fabio A, et al. Energy expenditure in children after severe traumatic brain injury. Pediatr Crit Care Med 2014;15:242-249.
- 8. Mehta NM, Bechard LJ, Dolan M, Ariagno K, Jiang H, et al. Energy imbalance and the risk of overfeeding in critically ill children. Pediatr Crit Care Med 2011;12:398-405.
- 9. Framson CM, LeLeiko NS, Dallal GE, Roubenoff R, Snelling LK, et al. Energy expenditure in critically ill children. Pediatr Crit Care Med 2007;8:264-267.
- 10. Havalad S, Quaid MA, Sapiega V. Energy expenditure in children with severe head injury: lack of agreement between measured and estimated energy expenditure. Nutr Clin Pract 2006;21:175-181.
- 11. Moore R, Najarian MP, Konvolinka CW. Measured energy expenditure in severe head trauma. J Trauma 1989;29:1633-1636.
- 12. World Health Organization: Energy and protein requirements: report of a joint FAO/WHO/UNU expert consultation. Geneva: World Health Organization; 1985, p 206.
- 13. Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 1985;39(Suppl 1):5-41.
- 14. Cote M, Lauzier F, Bibeau V, Labbe P, Turgeon AF. Nutritional support in severe traumatic brain injury. Crit Care 2011;15(Suppl 1):P375.
- 15. Kuzawa CW, Blair C. Metabolic costs and evolutionary implications of human brain development. Proc Natl Acad Sci U S A 2019;116:20657-20663.



