ABSTRACT
The present systematic review and meta-analysis were conducted in order to investigate the effects of capsinoids and fermented red pepper paste (FRPP) supplementation on lipid profile. Relevant studies were identified by searches of five databases from inception to November 2021 using relevant keywords. All clinical trials investigating the effect of capsinoids and FRPP on total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were included. Out of 1,203 citations, eight trials that enrolled 393 participants were included. Capsinoids and FRPP resulted in a significant reduction in TC (weighted mean differences [WMD], −9.92 mg/dL; 95% confidence interval [CI], −17.92 to −1.92; p = 0.015) but no significant changes in TG (WMD, −19.38 mg/dL; 95% CI, −39.94 to 1.18; p = 0.065), HDL-C (WMD, 0.83 mg/dL; 95% CI, −0.76 to 2.42; p = 0.305) and LDL-C (WMD, −0.59 mg/dL; 95% CI, −4.96 to 3.79; p = 0.793). Greater effects on TC were detected in trials performed on duration lasting less than twelve weeks, mean age of > 40, both sexes, and sample size of > 50. TG was reduced by using FRPP in studies conducted on mean age of > 40. HDL-C increased by using FRPP in studies conducted on duration of < 12 weeks, mean age of > 40, and sample size of ≤ 50. Overall, these data provided evidence that capsinoids and FRPP supplementation has beneficial effects on TC but not TG, HDL-C, and LDL-C.
-
Keywords: Lipid profile; Red pepper; Capsaicin; Meta-analysis
INTRODUCTION
Cardiovascular disease (CVD) is one of the most common chronic diseases in the world and one of the causes of disability and premature death. CVD mortality is predicted to increase to over 24 million a year by 2030 [
1,
2]. CVD includes coronary heart disease (CHD), strokes, peripheral arterial disease, and aortic disease [
3]. Often, advanced atherosclerosis results in CVD. Therefore, one way to prevent CVD is to control the factors that cause the hardening and narrowing of blood vessels and plaque build-up in vessels [
3,
4]. Data from various studies have shown that lipid concentrations are one of the major risk factors for atherosclerosis. Elevated circulating concentrations of plasma levels of triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and reduced levels of high-density lipoprotein cholesterol (HDL-C) increase the risk of CVD [
5,
6].
There are various pharmacological and non-pharmacological interventions to improve blood lipids. Statins are one of the most effective drugs for modifying LDL-C, TG, and HDL-C levels and therefore are used as the first-line drug for the treatment of dyslipidemia [
7]. Concomitant use of statins with compounds such as fibrate, niacin, or omega-3 fatty acids can optimize the process of lipid profile improvement; however, particular combination therapies can cause side effects and may not be tolerated [
7]. In addition, statins can be associated with serious side effects such as hepatotoxicity and myopathies [
8]. Replacing treatment with non-pharmacological and functional natural product compounds can prevent these complications.
Capsaicin (trans-8-methyl-N-vanillyl-6-nonenamide) is derived from homovanillic acid [
9]. The effective role of capsaicin-containing foods in health preservation has been expressed in traditional medicine for centuries [
10,
11]. This phytochemical is principally responsible for the pungent feeling of chili peppers. Because of its pungency, it causes
a burning sensations and relieves gastrointestinal side effects [
12]. Capsaicin is also offered as a food additive and medicinal compound [
9]. Clinical studies show that capsaicin supplementation has multiple pharmacologic and physiologic effects, including decreased appetite, increased body temperature and metabolism, improved postprandial hyperglycemia, hyperinsulinemia, and fasting lipid disorders. Its long-term supplementation is also effective in maintaining a reduced weight in obese participants. It also has analgesic and anti-cancer, anti-inflammatory, and antioxidant properties [
13,
14,
15]. Another benefit of capsaicin is its effects on the cardiovascular system. It increases the serum levels of HDL-C, decreases serum fasting triglyceride and ApoB levels, and inhibits phospholipid transfer protein (PLTP) activity [
15,
16,
17], although some studies have obtained conflicting results on lipid metabolism [
18]. Capsaicin also plays a part in the regulation of glucose metabolism and vascular function including the promotion of lipolysis by activating peroxisome proliferator-activated receptor delta (PPARδ) and the improvement of vasodilation by increasing endothelial nitric oxide synthase expression [
19,
20]. The anti-atherosclerotic property of capsaicin reduces the risk of CVD factors. Also, it can reduce waist: hip ratio and C-reactive protein [
14,
17]. Due to the well-known modulatory effects of capsaicin on inflammation and lipid metabolism, it is known as a cardioprotective agent [
17,
21,
22].
Because clinical studies of the effect of capsaicin or capsaicin-containing foods on lipid profile show contradictory effects, a meta-analysis of available evidence can provide a more accurate and precise estimate of the effect of capsinoids and fermented red pepper paste (FRPP) on lipid profile.
MATERIALS AND METHODS
The meta-analysis addressing the effects of capsinoids and FRPP supplementation on lipid profile was followed standard criteria (PRISMA criteria) for reporting meta-analyses [
23].
We searched the PubMed/MEDLINE, Scopus, ISI Web of Science, Embase, and Google Scholar up to November 2021. We used the following search strategy: (capsaicin OR capsaicinoid OR red pepper OR paprika OR chilli OR kochujang) AND (total cholesterol OR triglyceride OR low density lipoprotein OR high density lipoprotein OR blood lipids OR TC OR TG OR LDL-C OR HDL-C). We used applied relevant search terms to find a published English study (
Supplementary Table 1). We searched the references of the retrieved reports for any additional studies.
The search terms and strategies were constructed according to the PICOS model [
24]. Potentially relevant studies were considered for inclusion in this meta-analysis if they met the following inclusion criteria: age (adults aged 18 years old or more), intervention (capsinoids and FRPP), comparator (placebo), outcome (TC, TG, HDL-C, and LDL-C), and study design (parallel and cross-over clinical trial).
Studies were included if they met the following criteria: 1) randomized clinical trials (RCTs) comparing the effect of capsinoids or FRPP and placebo; 2) involved adults; 3) reported mean and standard deviation (SD) of TC, TG, HDL-C, and LDL-C. Studies were excluded if they were: 1) animal and in vitro studies; 2) uncontrolled trials; 3) without sufficient data 4) studies investigated the effects of capsinoids supplementation in composition with other interventions. We also excluded unpublished studies, letters, comments, conference papers, and observational studies.
Data extraction
Using a standardized form, pairs of authors (FS, MRA) independently reviewed the title and abstract of all articles and extracted the required data, assessed the quality of studies, and performed double-check. Any disagreements were resolved by a third investigator (AH). For each publication, we extracted the following information: name of first author, year of publication, study location, study design, trial duration, body mass index (BMI), and intervention (type, dose, and duration of supplementation); subjects’ information, including inclusion criteria, age, sex, health status, and BMI; outcomes assessed, including baseline and final values of outcomes (TC, TG, HDL-C, and LDL-C).
Quality assessment of studies
Study quality was assessed with the revised Cochrane risk-of-bias tool (RoB 2) [
25], which contains the following methodological domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome evaluation, uncompleted outcome data, selective reporting, and other potential threats to validity. According to the Cochrane Handbook recommendation, studies were stratified as low risk of bias; high risk of bias, and some concerns. Of included studies, 2 trials [
15,
26] did deviate from the intended interventions and had missing outcome data (
Table 1) [
14,
15,
16,
17,
18,
26,
27,
28].
Table 1Risk of bias for randomized controlled trials, assessed according to the revised Cochrane risk-of-bias tool for randomized trials (RoB 2)
Table 1
|
Publications |
Randomization process |
Deviations from the intended interventions |
Missing outcome data |
Measurement of the outcome |
Selection of the reported result |
Overall bias |
|
1. Kim (2010) [27] |
L |
L |
L |
L |
L |
L |
|
2. Cha (2013) [16] |
L |
L |
L |
L |
L |
L |
|
3. Lim (2015) [18] |
L |
S |
L |
L |
L |
L |
|
4. Yuan (2016) [15] |
L |
S |
S |
L |
L |
H |
|
5. Lee (2017) [26] |
L |
S |
S |
L |
L |
H |
|
6. Qin (2017) [17] |
L |
L |
L |
L |
L |
L |
|
7. Urbina (2017) [14] |
L |
L |
L |
L |
L |
L |
|
8. Kakutani (2018) [28] |
L |
L |
L |
L |
L |
L |
Statistical analysis
Treatment response was quantified using the mean changes and SDs in lipid profile including TC, TG, HDL-C, and LDL-C from baseline and standard mean difference, and 95% confidence interval (CI) were calculated to pool data and measure effect sizes in both intervention and control groups. When no SDs were reported for net change in lipid profile, the following formula was used: SD
difference = square root [(SD
pre-treatment)
2 + (SD
post-treatment)
2 − (2 × R × SD
pre-treatment × SD
post-treatment)], assuming a correlation coefficient (R) 0.8 as it is a conservative estimate for an expected range of 0–1 [
29]. When means (± SD) of outcome measures was not directly available and a standard error of the mean (SEM) was presented in place of SD, we converted it to SD using this formula: SD = SEM × √n, being “n” the number of subjects in each group. If medians and interquartile range were reported, mean and SD values were estimated using the method described by Hozo et al. [
30]. Ultimately, we used the GetData Graph Digitizer version 2.24 to extract data from studies that reported outcomes in the graphical form [
31].
I
2 testing performed to quantify the magnitude of inter-study heterogeneity and a value of I
2 more than 50% was considered as significant heterogeneity. The analyses were conducted using a random-effects meta-analysis [
32]. A priori subgroup analysis of dosage and duration of supplementation, supplement type, mean age, and the sample size was performed to detect potential sources of heterogeneity. Publication bias was examined by visually inspecting at funnel plots and quantitatively assessed using Egger’s test. In the case of substantial publication bias, the trim-and fill method was applied to regulate analysis for the impacts of publication bias [
33]. Meta-analysis was undertaken using Stata software version 14 (Stata Corp., College Station, TX, USA). The p values < 0.05 were considered to be statistically significant.
RESULTS
Study selection
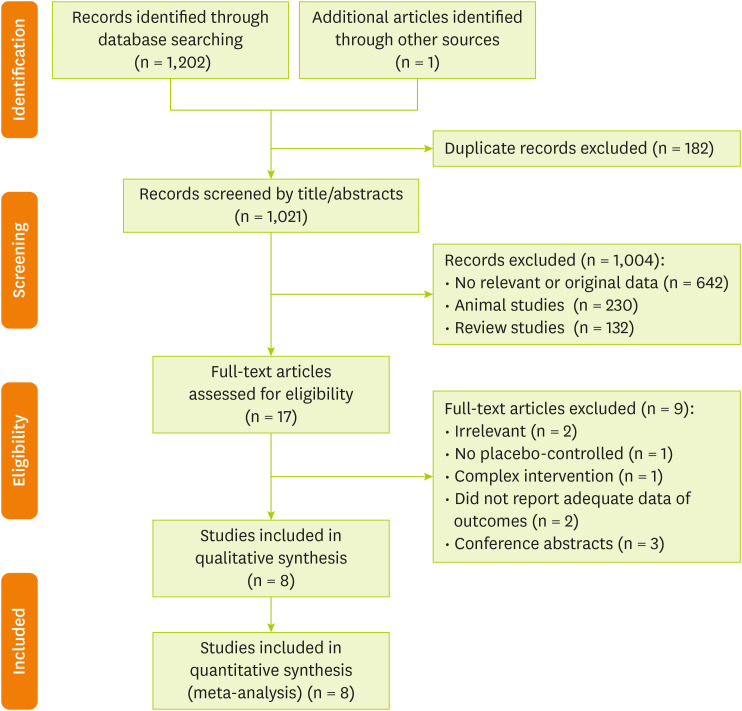
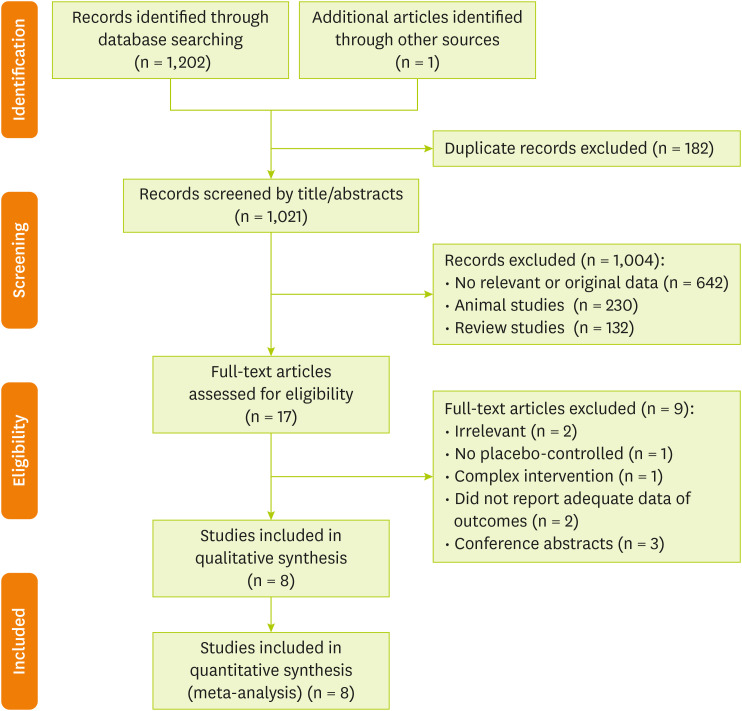
In total, 1,203 publications were identified in the initial search. After excluding duplicate papers (182) and unrelated studies (1,021), 17 papers were retained for full-text review. One additional trial was extracted through hand-searching of reference lists of related reviews. Of these articles, we excluded 9 publications due to the following reasons: irrelevant (n = 2), has no placebo-controlled group (n = 1), complex intervention (n = 1), without sufficient data for outcomes (n = 2) and conference abstracts (n = 3). Finally, 8 studies met all our inclusion criteria. The A flow diagram of the study selection is provided in
Figure 1.
Figure 1Flow chart of the number of studies identified and selected into the meta-analysis.
Characteristics of included studies
The characteristics of studies included in the systematic review and meta-analysis are provided in
Table 2 [
14,
15,
16,
17,
18,
26,
27,
28]. Included trials enrolled 393 participants with an age range between 20 to 43.5 years old and were conducted on both sexes. Four trials were conducted in Korea [
16,
18,
26,
27], Japan [
28], China [
15,
17], and US [
14], which involved 393 participants with different health statuses, such as healthy [
14,
27,
28], overweight/obese [
26], overweight [
16], hyperlipidemia [
18], adults with low HDL-C [
17], and gestational diabetes mellitus (GDM) [
15]. Publication dates ranged from 2010 to 2018. Eight of the included studies had parallel designs. Among the included studies, six studies included men and women [
14,
15,
17,
18,
26,
28] except for three studies that were included only women [
15,
27,
28], and one study was performed on men [
28]. Three studies on kochujang [
16,
18,
26], two studies on capsaicin [
15,
17], one study on fermented red pepper paste [
27], one study on capsinoid [
14], and one study on paprika xanthophylls [
28] were included. All studies that tested red pepper used a dose of 11.9 g [
16,
18,
26,
27]. Also, capsinoids were administrated in doses ranging from 2 [
14] to 9 mg/day [
28], and the intervention duration was from 3 to 12 weeks. Out of eight studies, LDL-C, HDL-C, TG, and TC were reported in eight [
14,
15,
16,
17,
18,
26,
27,
28], seven [
14,
15,
16,
17,
18,
26,
28], seven [
14,
15,
16,
17,
18,
26,
28] and seven studies [
15,
16,
17,
18,
26,
27,
28], respectively.
Table 2Demographic characteristics of the included studies
Table 2
|
First author (year) |
Location |
Study design |
Health status |
Sex |
Sample size |
Duration (week) |
Mean age (year) |
Baseline BMI (kg/m2) |
Intervention |
Outcome |
|
Treatment group |
Control group |
|
Kim (2010) [27] |
Korea |
Randomized, double-blind, placebo-controlled, parallel trial |
Healthy |
Female |
28 |
12 |
39.5 |
23 < |
11.9 g FRPP TC/LDL-unvaried group |
Placebo |
LDL/TC |
|
Cha (2013) [16] |
Korea |
Randomized, double-blind, placebo-controlled, parallel trial |
Overweight |
Both |
60 |
12 |
42 |
23 ≤ |
Kochujang (11.9 g red pepper) |
Placebo |
TC/LDL/HDL/TG |
|
Lim (2015) [18] |
Korea |
Randomized, double-blind, placebo-controlled, parallel trial |
Hyperlipidemia |
Both |
30 |
12 |
42 |
26.9 |
Kochujang (11.9 g red pepper) |
Placebo |
TC/LDL/HDL/TG |
|
Yuan (2016) [15] |
China |
Randomized, double-blind, placebo-controlled, parallel trial |
GDM |
Female |
44 |
4 |
30.45 |
27 |
5 mg capsaicin |
Placebo |
TC/LDL/HDL/TG |
|
Lee (2017) [26] |
Korea |
Randomized, double-blind, placebo-controlled, parallel trial |
Overweight/Obese |
Both |
60 |
12 |
42 |
23 ≤ |
Kochujang (11.9 g red pepper) |
Placebo |
TC/LDL/HDL/TG |
|
Qin (2017) [17] |
China |
Randomized, double-blind, placebo-controlled, parallel trial |
Adults with low HDL-C |
Both |
42 |
3 |
43.55 |
26.2 |
4 mg capsaicin |
Placebo |
TC/LDL/HDL/TG |
|
Urbina (2017) [14] |
US |
Randomized, single-blind, placebo-controlled, parallel trial |
Healthy |
Both |
22 |
12 |
30 |
27.5 |
2 mg low-dose capsinoid |
Placebo |
LDL/HDL/TG |
|
Randomized, double-blind, placebo-controlled, parallel trial |
27 |
4 mg high-dose capsinoid |
|
Kakutani (2018) [28] |
Japan |
Randomized, double-blind, placebo-controlled, parallel trial |
Healthy |
Both |
80 |
12 |
20 |
27.5 |
9 mg paprika xanthophylls |
Placebo |
TC/LDL/TG |
|
Male |
67 |
HDL |
|
Female |
13 |
HDL |
Effect of pepper on plasma concentrations of TC
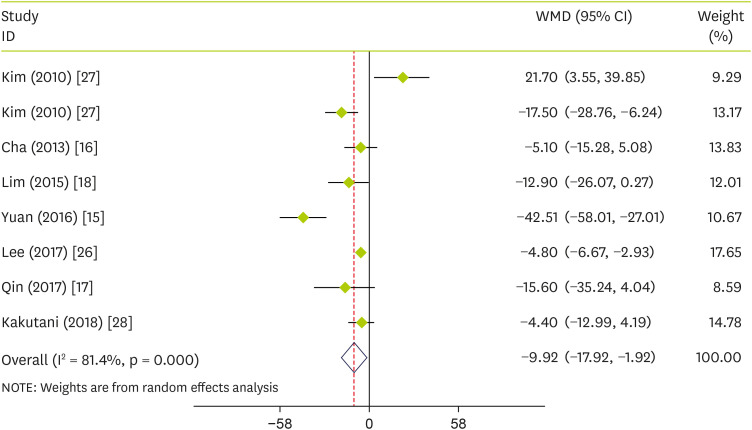
Combining data from 7 studies showed that pepper consumption significantly reduced TC (WMD, −9.92 mg/dL; 95% CI, −17.92 to −1.92; p = 0.015) (I
2 = 81.4%; p < 0.001) compared to the control group (
Figure 2). To find any source of heterogeneity, we performed subgroup analyses based on intervention type (capsinoids or FRPP), age (≤ 40 or > 40), sex (both or female), trial duration (< 12 week or ≥ 12 week) and sample size (≤ 50 or > 50). Subgroup analysis according to the intervention type also showed no effect of capsinoids (p = 0.102) and FRPP (p = 0.183) on TC. Dividing RCTs based on the aforementioned subgroups showed that TC was decreased significantly following pepper supplementation in those RCTs that were conducted on supplement duration lasted less than twelve weeks, the mean age of > 40, both sexes, and the sample size of > 50 (
Table 3).
Figure 2
Forest plot detailing WMD and 95% CIs for the effect of capsinoids and FRPP supplementation on TC.
WMD, weighted mean differences; CI, confidence interval; FRPP, fermented red pepper paste; TC, total cholesterol.
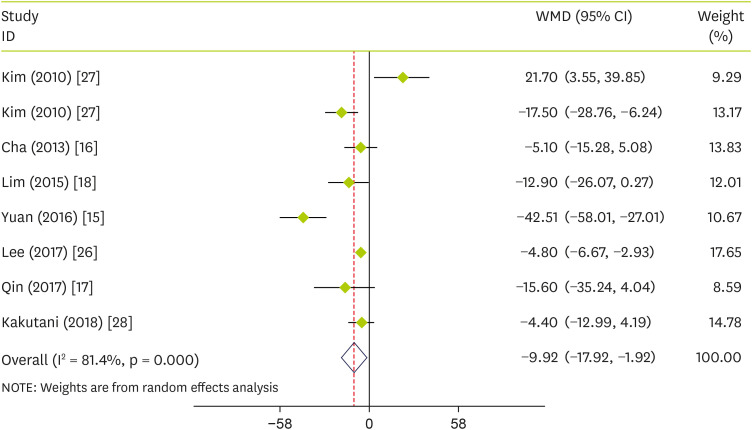
Table 3Subgroup analysis of included RCTs in meta-analysis of the effect of capsinoids and FRPP supplementation on lipid profile
Table 3
|
Group |
No. of trials |
WMD (95% CI) |
p value |
I2 (%) |
p-heterogeneity |
p for between subgroup heterogeneity |
|
TC |
|
|
|
|
|
|
|
Intervention |
|
|
|
|
|
0.020 |
|
|
Capsinoids |
3 |
−20.41 (−44.86, 4.03) |
0.102 |
88.8 |
< 0.001 |
|
|
FRPP |
4 |
−5.5 (−13.58, 2.59) |
0.183 |
72.3 |
0.006 |
|
Mean age (yr) |
|
|
|
|
|
0.007 |
|
|
≤ 40 |
2 |
−13.16 (−44.99, 18.68) |
0.418 |
92.8 |
< 0.001 |
|
|
> 40 |
5 |
−5.03 (−6.80, −3.25) |
< 0.001 |
0 |
0.633 |
|
Sex |
|
|
|
|
|
0.007 |
|
|
Both |
5 |
−5.03 (−6.80, −3.25) |
< 0.001 |
0 |
0.633 |
|
|
Female |
2 |
−13.16 (−44.99, 18.68) |
0.418 |
92.8 |
< 0.001 |
|
Duration (wk) |
|
|
|
|
|
< 0.001 |
|
|
< 12 |
2 |
−29.76 (−56.09, −3.42) |
0.027 |
77.5 |
0.035 |
|
|
≥ 12 |
5 |
−5.49 (−11.66, −0.69) |
0.082 |
65.5 |
0.013 |
|
Sample size |
|
|
|
|
|
0.002 |
|
|
≤ 50 |
4 |
−13.76 (−31.57, 4.05) |
0.130 |
85.7 |
< 0.001 |
|
|
> 50 |
3 |
−4.79 (−6.59, −3.00) |
< 0.001 |
0 |
0.994 |
|
TG |
|
|
|
|
|
|
|
Intervention |
|
|
|
|
|
< 0.001 |
|
|
Capsinoids |
4 |
−16.57 (−51.87, 18.72) |
0.357 |
88.8 |
< 0.001 |
|
|
FRPP |
3 |
−29.44 (−33.16, −25.72) |
< 0.001 |
0 |
0.706 |
|
Mean age (yr) |
|
|
|
|
|
< 0.001 |
|
|
≤ 40 |
2 |
−18.64 (−68.88, 31.60) |
0.467 |
94 |
< 0.001 |
|
|
> 40 |
5 |
−29.24 (−32.93, −25.55) |
< 0.001 |
0 |
0.802 |
|
Duration (wk) |
|
|
|
|
|
0.004 |
|
|
< 12 |
2 |
−52.04 (−140.33, 36.25) |
0.248 |
84.8 |
0.010 |
|
|
≥ 12 |
5 |
−10.50 (−31.61, 10.61) |
0.329 |
89.5 |
< 0.001 |
|
Sample size |
|
|
|
|
|
0.039 |
|
|
≤ 50 |
3 |
−38.73 (−101.50, 24.03) |
0.226 |
82.2 |
0.004 |
|
|
> 50 |
4 |
−10.50 (−33.37, 12.37) |
0.368 |
91.5 |
< 0.001 |
|
HDL |
|
|
|
|
|
|
|
Intervention |
|
|
|
|
|
0.945 |
|
|
Capsinoids |
4 |
−0.09 (−3.41, 3.22) |
0.956 |
65.2 |
0.013 |
|
|
FRPP |
3 |
1.63 (1.06, 2.20) |
< 0.001 |
0 |
0.509 |
|
Mean age (yr) |
|
|
|
|
|
0.032 |
|
|
≤ 40 |
2 |
−1.54 (−5.73, 2.64) |
0.470 |
57 |
0.098 |
|
|
> 40 |
5 |
1.79 (0.59, 3.00) |
0.003 |
22.7 |
0.263 |
|
Sex |
|
|
|
|
|
0.862 |
|
|
Both |
5 |
0.62 (−1.34, 2.59) |
0.533 |
65.9 |
0.012 |
|
|
Male |
1 |
−0.50 (−8.31, 7.31) |
0.900 |
- |
- |
|
|
Female |
2 |
1.48 (−2.36, 5.31) |
0.450 |
0 |
0.383 |
|
Duration (wk) |
|
|
|
|
|
0.036 |
|
|
< 12 |
2 |
3.59 (1.69, 5.50) |
< 0.001 |
0 |
0.524 |
|
|
≥ 12 |
5 |
−0.37 (−2.40, 1.66) |
0.722 |
45.1 |
0.090 |
|
Sample size |
|
|
|
|
|
0.104 |
|
|
≤ 50 |
3 |
2.67 (0.30, 5.04) |
0.027 |
28.6 |
0.246 |
|
|
> 50 |
4 |
−0.51 (−2.86, 1.84) |
0.673 |
51.4 |
0.068 |
|
LDL |
|
|
|
|
|
|
|
Intervention |
|
|
|
|
|
0.138 |
|
|
Capsinoids |
4 |
2.56 (−1.64, 6.77) |
0.233 |
0 |
0.892 |
|
|
FRPP |
4 |
−3.74 (−12.60, 5.11) |
0.408 |
81.3 |
< 0.001 |
|
Mean age (yr) |
|
|
|
|
|
0.833 |
|
|
≤ 40 |
3 |
1.08 (−9.25, 11.41) |
0.838 |
74.1 |
0.004 |
|
|
> 40 |
5 |
−1.16 (−5.82, 3.49) |
0.624 |
56.5 |
0.056 |
|
Sex |
|
|
|
|
|
0.500 |
|
|
Both |
6 |
−0.15 (−3.94, 3.64) |
0.937 |
42.9 |
0.105 |
|
|
Female |
2 |
−0.61 (−18.87, 17.64) |
0.948 |
85.4 |
0.001 |
|
Duration (wk) |
|
|
|
|
|
0.304 |
|
|
< 12 |
2 |
2.83 (−3.49, 9.15) |
0.380 |
0 |
0.435 |
|
|
≥ 12 |
6 |
−1.42 (−6.87, 4.02) |
0.608 |
69.6 |
0.002 |
|
Sample size |
|
|
|
|
|
0.282 |
|
|
≤ 50 |
4 |
−2.45 (−13.77, 8.87) |
0.671 |
82 |
< 0.001 |
|
|
> 50 |
4 |
−0.06 (−1.76, 1.65) |
0.948 |
0 |
0.867 |
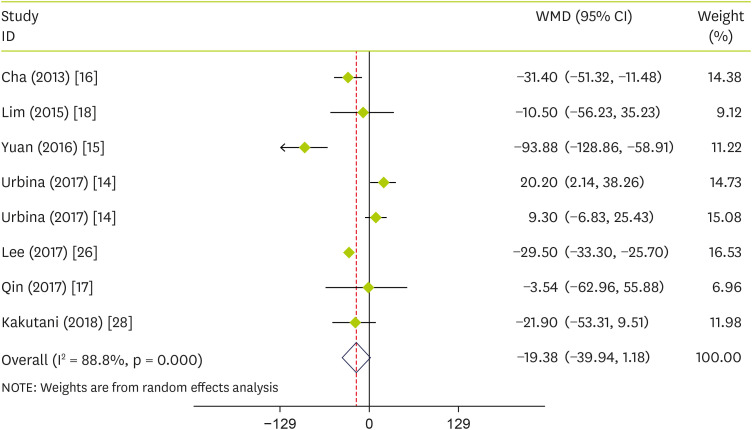
Effect of pepper on plasma concentrations of TG
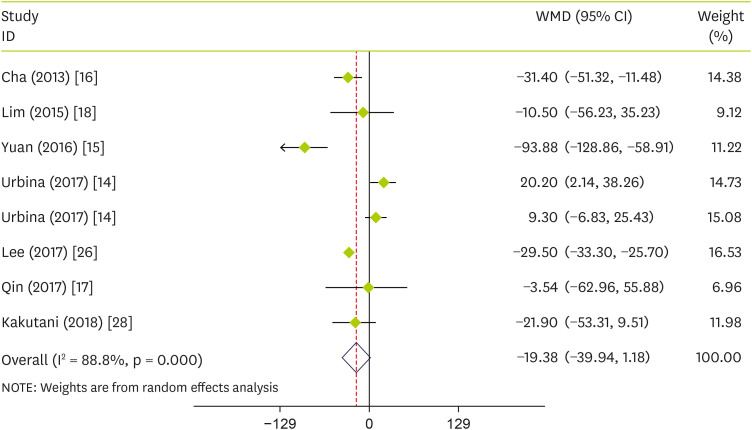
In the pooled analysis of seven trials, there was no significant pooled effects of pepper consumption on TG (WMD, −19.38 mg/dL; 95% CI, −39.94 to 1.18; p = 0.065) (I
2 = 88.8%; p < 0.001) (
Figure 3). Subgroup analysis according to the intervention type observed that pepper significantly reduced TG in studies conducted on FRPP (WMD, −29.44; 95% CI, −33.16 to −25.72; p < 0.001) and mean age of > 40 (WMD, −29.24; 95% CI, −32.93 to −25.55; p < 0.001) (
Table 3).
Figure 3
Forest plot detailing WMD and 95% CIs for the effect of capsinoids and FRPP supplementation on TG.
WMD, weighted mean differences; CI, confidence interval; FRPP, fermented red pepper paste; TG, triglycerides.
Effect of pepper on plasma concentrations of HDL-C
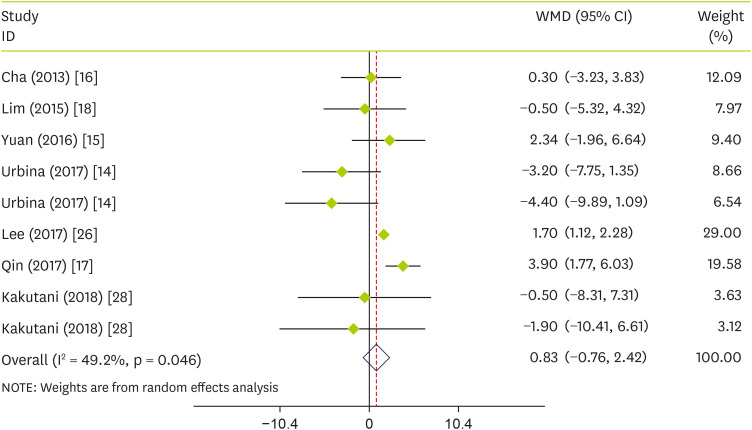
Pooled effect sizes indicated that pepper consumption had no significant effect on HDL-C (WMD, 0.83 mg/dL; 95% CI, −0.76 to 2.42; p = 0.305) (I
2 = 49.2%, p = 0.046) (
Figure 4). Stratified analysis showed that pepper significantly increases HDL-C in studies conducted on FRPP (WMD, 1.63; 95% CI, 1.06 to 2.20; p < 0.001), mean age of > 40 (WMD, 1.79; 95% CI, 0.59 to 3.00; p = 0.003), duration lasting less than twelve weeks (WMD, 3.59; 95% CI, 1.69 to 5.50; p = 0.003), and sample size of ≤ 50 (WMD, 2.67; 95% CI, 0.30 to 5.04; p = 0.003) (
Table 3).
Figure 4
Forest plot detailing WMD and 95% CIs for the effect of capsinoids and FRPP supplementation on HDL-C.
WMD, weighted mean differences; CI, confidence interval; FRPP, fermented red pepper paste; HDL-C, high-density lipoprotein cholesterol.

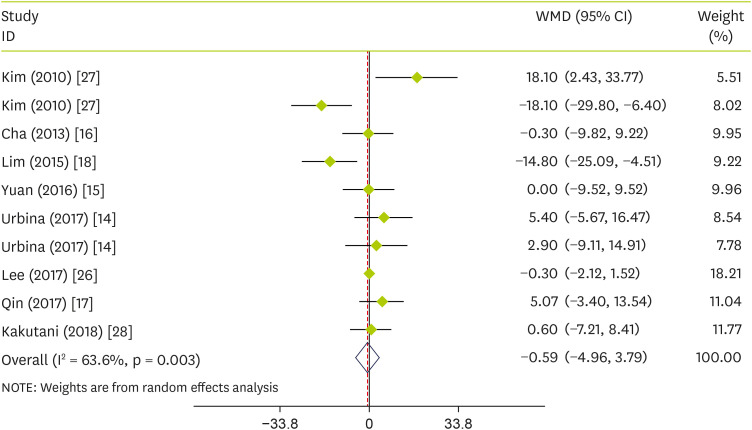
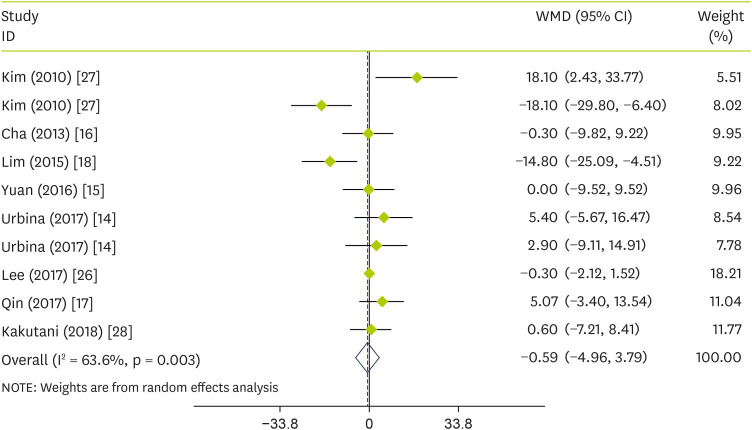
Effect of pepper on plasma concentrations of LDL-C
The pooled analysis showed that the effect of pepper consumption on LDL-C was not significant (WMD, −0.59 mg/dL; 95% CI, −4.96 to 3.79; p = 0.793) (I
2 = 63.6%; p = 0.003). The effect of pepper consumption on LDL-C is shown in
Figure 5. Stratified analyses were performed based on intervention type, supplementation duration, age, sex, and sample size. Findings showed that none of these variables could explain the inter-study heterogeneity (
Table 3).
Figure 5
Forest plot detailing WMD and 95% CIs for the effect of capsinoids and FRPP supplementation on LDL-C.
WMD, weighted mean differences; CI, confidence interval; FRPP, fermented red pepper paste; LDL-C, low-density lipoprotein cholesterol.
Publication bias and sensitivity analysis
In sensitivity analyses in which we excluded any single study at a time from each analysis the overall estimates were not substantially altered. There was no evidence of publication bias with Egger’s weighted regression test for TC (p = 0.370), TG (p = 0.473), HDL-C (p = 0.169), and LDL-C (p = 0.967).
DISCUSSION
Based on current knowledge, patients with hypercholesterolemia experience a significant increase in atherosclerotic cardiovascular disease (ASCVD) as well as CHD [
34,
35,
36]. Epidemiological studies have shown that there is a significant difference in blood lipid profile in Asian people compared to people living in western countries. Lower harmful blood lipids have an effective role in reducing cardiovascular disease-related diseases. The eating habit of Asian people is one of the main factors that reduce harmful fats in their lipid profiles. As we know, the main feature of Asian foods is the presence of special spices such as chili and other spices [
18]. In this study, we hypothesized that capsaicin improves lipid profiles among adults. Our findings showed that capsaicin has a reducing effect on TC, and no significant effects on other lipid profiles such as TG, LDL-C and HDL-C. To our knowledge, the current study is the first meta-analysis of RCTs on the effects of capsinoids on lipid profile.
We included a total of 8 RCTs that examined the effect of capsinoids on lipid profiles among adults and found that capsaicin may have beneficial effects on reducing lipid profile components. The evidence from human studies indicates that capsaicin in the form of an extract or supplement reduces the serum levels of TG and increases the serum levels of HDL-C. Moreover, some studies have shown a positive effect of capsaicin on body weight and body fat. In contrast, other studies have found no significant effect on the body weight, body fat percentage, BMI, and lipid profile of participants. These contradictory results might be explained by type of intervention, capsaicin dose, age, and sex of the subjects studied. Studies in humans have been conducted using a wide range of doses of pepper (varying from daily intake to a continuous dose of up to 12 weeks) [
14,
16,
17,
18,
26]. The controversial findings of studies investigating the effect of capsaicin on lipid profile raise the possibility that some determinants have not been sufficiently addressed. One study on capsaicin was short-term [
15]; therefore, it may not be able to provide accurate information on the change in lipid profile. Furthermore, long-duration daily consumption (12 weeks) of 34.5 g aspergillus oryzae-fermented kochujang (fermented soybean-based red pepper paste) has shown that capsaicin is beneficial for decreasing LDL-C and TC levels in subjects with hyperlipidemia [
18]. However, it seems that the effects of capsaicin are different in a healthy adult.
Indeed, Yuan et al. [
15] reported that supplementation of capsaicin-containing chili for 4 weeks has beneficial effects on regulating lipid metabolism; it improved fasting lipid metabolic disorders in women with GDM. Qin et al. indicated that daily consumption of a 4 mg capsaicin supplement for three months effectively increased the levels of fasting serum HDL-C in adults with low HDL-C [
17]. This increase was about 0.08 mmol/L (3 mg/dL); as we know, an increase of about 3 mg/L of HDL-C is clinically significant [
15,
17,
37,
38]. In another study by Yuan et al. [
15], reports indicate that consumption of capsaicin-containing chili powder has positive effects on serum TG levels in women with gestational diabetes mellitus. The duration of this study was four weeks and capsaicin-containing chili powder significantly decreased the TG level from 3.67 to 2.75 mmol/L in these women [
15]. According to Qin et al.’s study [
17], in people with low HDL-C, capsaicin improves risk factors for CHD and may be used to treat and prevent CHD [
17]. Consumption of capsaicin or red pepper paste is probably able to reduce lipid disorders, while it does not affect normal fat metabolism [
17].
It has also been shown that capsaicin reduces cardiac fibrosis and hypertrophy caused by hypertension [
20,
39]. One of the limitations of our study lies in the fact that the mechanisms underlying the metabolic effects of capsaicin on human lipid profiles are not fully understood. The possible mechanisms of action of capsaicin supplementation on HDL-C levels in adults, based on findings of the study concluded by Kang et al. [
40] may be associated with the anti-inflammatory effects of capsaicin. Also, this study suggested that the beneficial effects of capsaicin in individuals with low HDL-C levels may be associated with a change in their gut microbiota [
40]. Another proposed mechanism for capsaicin’s effect on blood lipids involves a significant reduction in the activity of PLTP when supplemented with capsaicin, whereas cholesterol transfer protein and cholesterol-acyl di-lecithin transferase (LCAT) were not significantly different in concentration and activity [
41,
42]. Additionally, increased plasma PLTP activity is an indicator of CVD [
43] and has a positive relationship with left ventricular systolic dysfunction in patients with CHD [
44] and carotid intima thickness in type 2 diabetics [
45]. In humans with CHD or premenopausal women, plasma PLTP activity is inversely related to HDL-C levels [
17,
46].
Although the present study was the first to investigat
ed the effects of capsinoids supplementation on lipid profile, this meta-analysis has several important limitations. Our study was limited by a lack of understanding of how capsaicin affects the lipid profile. Another limitation of this study is the lack of an adequate number of eligible RCTs. Also, these RCTs did not have long-term effects and had a small sample size. Thus, larger target groups and longer interventions are needed in future studies. Statistical analysis was also limited by the increased risk of Type I errors due to testing multiple study outcomes. Atherosclerosis is associated with high blood lipid concentrations, as previously mentioned. A reduction in HDL-C and increases in TG, TC, and LDL-C can cause CVD [
7]. To improve blood lipids, there are a variety of pharmaceutical and non-pharmacological interventions. Generally, drug treatments are preferred over non-drug treatments for improving LDL-C, TG, and HDL-C levels. However, drugs like statins can have serious side effects, such as liver toxicity and myopathy [
8]. Therefore, development of alternatives to drugs can be crucial in reducing the side effects caused by drugs in this situation. One of the recommended and alternative treatments was the capsinoids and fermented red pepper paste supplementation, which we discussed in this study.
In summary, the main conclusion of our meta-analysis is that capsinoids significantly reduce the serum levels of TC but have no significant effect on other components of the lipid profile.
NOTES
-
Conflict of Interest: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Amini MR.
Data curation: Amini MR.
Formal analysis: Amini MR.
Methodology: Mohtashaminia F.
Project administration: Hekmatdoost A.
Supervision: Hekmatdoost A.
Writing - original draft: Payandeh N, Alvani M, Talebyan A.
Writing - review & editing: Sheikhhossein F, Amini MR.
ACKNOWLEDGEMENTS
This study is related to the project NO. 1400/65021 From Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
We also appreciate the “Student Research Committee” and “Research & Technology Chancellor” in Shahid Beheshti University of Medical Sciences for their financial support of this study.
SUPPLEMENTARY MATERIAL
REFERENCES
- 1. Sarrafzadegan N, Mohammmadifard N. Cardiovascular disease in Iran in the last 40 years: prevalence, mortality, morbidity, challenges and strategies for cardiovascular prevention. Arch Iran Med 2019;22:204-210.
- 2. Sheet-Populations SF. International cardiovascular disease statistics. Dallas (TX): American Heart Association; 2004.
- 3. Bhupathi V, Mazariegos M, Cruz Rodriguez JB, Deoker A. Dairy intake and risk of cardiovascular disease. Curr Cardiol Rep 2020;22:11.
- 4. Sahebkar A. A systematic review and meta-analysis of randomized controlled trials investigating the effects of curcumin on blood lipid levels. Clin Nutr 2014;33:406-414.
- 5. Assmann G. Dyslipidaemia and global cardiovascular risk: clinical issues. Eur Heart J Suppl 2006;8:F40-F46.
- 6. Musunuru K. Atherogenic dyslipidemia: cardiovascular risk and dietary intervention. Lipids 2010;45:907-914.
- 7. Gaw A. HDL-C and triglyceride levels: relationship to coronary heart disease and treatment with statins. Cardiovasc Drugs Ther 2003;17:53-62.
- 8. Padala S, Thompson PD. Statins as a possible cause of inflammatory and necrotizing myopathies. Atherosclerosis 2012;222:15-21.
- 9. Chapa-Oliver AM, Mejía-Teniente L. Capsaicin: from plants to a cancer-suppressing agent. Molecules 2016;21:931.
- 10. Cichewicz RH, Thorpe PA. The antimicrobial properties of chile peppers (Capsicum species) and their uses in Mayan medicine. J Ethnopharmacol 1996;52:61-70.
- 11. Yamamoto S, Nawata E. Use of Capsicum frutescens L. by the indigenous peoples of Taiwan and the Batanes Islands. Econ Bot 2009;63:43-59.
- 12. Lejeune MP, Kovacs EM, Westerterp-Plantenga MS. Effect of capsaicin on substrate oxidation and weight maintenance after modest body-weight loss in human subjects. Br J Nutr 2003;90:651-659.
- 13. Rigamonti AE, Casnici C, Marelli O, De Col A, Tamini S, Lucchetti E, Tringali G, De Micheli R, Abbruzzese L, Bortolotti M, Cella SG, Sartorio A. Acute administration of capsaicin increases resting energy expenditure in young obese subjects without affecting energy intake, appetite, and circulating levels of orexigenic/anorexigenic peptides. Nutr Res 2018;52:71-79.
- 14. Urbina SL, Roberts MD, Kephart WC, Villa KB, Santos EN, Olivencia AM, Bennett HM, Lara MD, Foster CA, Purpura M, Jäger R, Taylor LW, Wilborn CD. Effects of twelve weeks of capsaicinoid supplementation on body composition, appetite and self-reported caloric intake in overweight individuals. Appetite 2017;113:264-273.
- 15. Yuan LJ, Qin Y, Wang L, Zeng Y, Chang H, Wang J, Wang B, Wan J, Chen SH, Zhang QY, Zhu JD, Zhou Y, Mi MT. Capsaicin-containing chili improved postprandial hyperglycemia, hyperinsulinemia, and fasting lipid disorders in women with gestational diabetes mellitus and lowered the incidence of large-for-gestational-age newborns. Clin Nutr 2016;35:388-393.
- 16. Cha YS, Kim SR, Yang JA, Back HI, Kim MG, Jung SJ, Song WO, Chae SW. Kochujang, fermented soybean-based red pepper paste, decreases visceral fat and improves blood lipid profiles in overweight adults. Nutr Metab (Lond) 2013;10:24.
- 17. Qin Y, Ran L, Wang J, Yu L, Lang HD, Wang XL, Mi MT, Zhu JD. Capsaicin supplementation improved risk factors of coronary heart disease in individuals with low HDL-C levels. Nutrients 2017;9:9.
- 18. Lim JH, Jung ES, Choi EK, Jeong DY, Jo SW, Jin JH, Lee JM, Park BH, Chae SW. Supplementation with Aspergillus oryzae-fermented kochujang lowers serum cholesterol in subjects with hyperlipidemia. Clin Nutr 2015;34:383-387.
- 19. Panchal SK, Bliss E, Brown L. Capsaicin in metabolic syndrome. Nutrients 2018;10:630.
- 20. Sun F, Xiong S, Zhu Z. Dietary capsaicin protects cardiometabolic organs from dysfunction. Nutrients 2016;8:174.
- 21. Sharma SK, Vij AS, Sharma M. Mechanisms and clinical uses of capsaicin. Eur J Pharmacol 2013;720:55-62.
- 22. Srinivasan K. Biological activities of red pepper (Capsicum annuum) and its pungent principle capsaicin: a review. Crit Rev Food Sci Nutr 2016;56:1488-1500.
- 23. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1.
- 24. Richardson WS, Wilson MC, Nishikawa J, Hayward RS. The well-built clinical question: a key to evidence-based decisions. ACP J Club 1995;123:A12-A13.
- 25. Higgins JPT, Altman DG. Chapter 8. Assessing risk of bias in included studies. In Higgins JPT, Green S, eds, ddCochrane handbook for systematic reviews of interventions: Cochrane book series. Hoboken (NJ): Wiley-Blackwell; 2008, pp 187-241.
- 26. Lee Y, Cha YS, Park Y, Lee M.
PPARγ2 C1431T polymorphism interacts with the antiobesogenic effects of kochujang, a Korean fermented, soybean-based red pepper paste, in overweight/obese subjects: a 12-week, double-blind randomized clinical trial. J Med Food 2017;20:610-617.
- 27. Kim Y, Park YJ, Yang SO, Kim SH, Hyun SH, Cho S, Kim YS, Kwon DY, Cha YS, Chae S, Choi HK. Hypoxanthine levels in human urine serve as a screening indicator for the plasma total cholesterol and low-density lipoprotein modulation activities of fermented red pepper paste. Nutr Res 2010;30:455-461.
- 28. Kakutani R, Hokari S, Nishino A, Ichihara T, Sugimoto K, Takaha T, Kuriki T, Maoka T. Effect of oral paprika xanthophyll intake on abdominal fat in healthy overweight humans: a randomized, double-blind, placebo-controlled study. J Oleo Sci 2018;67:1149-1162.
- 29. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Hoboken (NJ): John Wiley & Sons; 2009.
- 30. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13.
- 31. Fedorov S. GetData Graph Digitizer version 2.24. Get data-graph-digitizer-com. Moscow: GetData Graph Digitizer; 2002.
- 32. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007;28:105-114.
- 33. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455-463.
- 34. Grundy SM. Management of high serum cholesterol and related disorders in patients at risk for coronary heart disease. Am J Med 1997;102:15-22.
- 35. Nyiambam W, Sylverken AA, Owusu IK, Buabeng KO, Boateng FA, Owusu-Dabo E. Cardiovascular disease risk assessment among patients attending two cardiac clinics in the Ashanti region of Ghana. Ghana Med J 2020;54:140-145.
- 36. Satoh M, Ohkubo T, Asayama K, Murakami Y, Sugiyama D, Waki T, Tanaka-Mizuno S, Yamada M, Saitoh S, Sakata K, Irie F, Sairenchi T, Ishikawa S, Kiyama M, Okayama A, Miura K, Imai Y, Ueshima H, Okamura T. Evidence for Cardiovascular Prevention from Observational Cohorts in Japan (EPOCH–JAPAN) Research Group. A combination of blood pressure and total cholesterol increases the lifetime risk of coronary heart disease mortality: EPOCH-JAPAN. J Atheroscler Thromb 2021;28:6-24.
- 37. Di Angelantonio E, Gao P, Pennells L, Kaptoge S, Caslake M, Thompson A, Butterworth AS, Sarwar N, Wormser D, Saleheen D, Ballantyne CM, Psaty BM, Sundström J, Ridker PM, Nagel D, Gillum RF, Ford I, Ducimetiere P, Kiechl S, Koenig W, Dullaart RP, Assmann G, D’Agostino RB Sr, Dagenais GR, Cooper JA, Kromhout D, Onat A, Tipping RW, Gómez-de-la-Cámara A, Rosengren A, Sutherland SE, Gallacher J, Fowkes FG, Casiglia E, Hofman A, Salomaa V, Barrett-Connor E, Clarke R, Brunner E, Jukema JW, Simons LA, Sandhu M, Wareham NJ, Khaw KT, Kauhanen J, Salonen JT, Howard WJ, Nordestgaard BG, Wood AM, Thompson SG, Boekholdt SM, Sattar N, Packard C, Gudnason V, Danesh J. Emerging Risk Factors Collaboration. Lipid-related markers and cardiovascular disease prediction. JAMA 2012;307:2499-2506.
- 38. Ludy MJ, Moore GE, Mattes RD. The effects of capsaicin and capsiate on energy balance: critical review and meta-analyses of studies in humans. Chem Senses 2012;37:103-121.
- 39. Yang D, Luo Z, Ma S, Wong WT, Ma L, Zhong J, He H, Zhao Z, Cao T, Yan Z, Liu D, Arendshorst WJ, Huang Y, Tepel M, Zhu Z. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab 2010;12:130-141.
- 40. Kang C, Wang B, Kaliannan K, Wang X, Lang H, Hui S, Huang L, Zhang Y, Zhou M, Chen M, Mi M. Gut microbiota mediates the protective effects of dietary capsaicin against chronic low-grade inflammation and associated obesity induced by high-fat diet. MBio 2017;8:e00470-17.
- 41. Zannis VI, Chroni A, Krieger M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med (Berl) 2006;84:276-294.
- 42. Quintão EC, Cazita PM. Lipid transfer proteins: past, present and perspectives. Atherosclerosis 2010;209:1-9.
- 43. Vergeer M, Boekholdt SM, Sandhu MS, Ricketts SL, Wareham NJ, Brown MJ, de Faire U, Leander K, Gigante B, Kavousi M, Hofman A, Uitterlinden AG, van Duijn CM, Witteman JC, Jukema JW, Schadt EE, van der Schoot E, Kastelein JJ, Khaw KT, Dullaart RP, van Tol A, Trip MD, Dallinga-Thie GM. Genetic variation at the phospholipid transfer protein locus affects its activity and high-density lipoprotein size and is a novel marker of cardiovascular disease susceptibility. Circulation 2010;122:470-477.
- 44. Cavusoglu E, Marmur JD, Chhabra S, Chopra V, Eng C, Jiang XC. Relation of baseline plasma phospholipid transfer protein (PLTP) activity to left ventricular systolic dysfunction in patients referred for coronary angiography. Atherosclerosis 2009;207:261-265.
- 45. de Vries R, Dallinga-Thie GM, Smit AJ, Wolffenbuttel BH, van Tol A, Dullaart RP. Elevated plasma phospholipid transfer protein activity is a determinant of carotid intima-media thickness in type 2 diabetes mellitus. Diabetologia 2006;49:398-404.
- 46. Murdoch SJ, Carr MC, Hokanson JE, Brunzell JD, Albers JJ. PLTP activity in premenopausal women. Relationship with lipoprotein lipase, HDL, LDL, body fat, and insulin resistance. J Lipid Res 2000;41:237-244.
 , Nastaran Payandeh2
, Nastaran Payandeh2 , Fatemeh Sheikhhossein3
, Fatemeh Sheikhhossein3 , Mohsen Alvani4
, Mohsen Alvani4 , Alireza Talebyan5
, Alireza Talebyan5 , Fatemeh Mohtashaminia2
, Fatemeh Mohtashaminia2 , Azita Hekmatdoost6
, Azita Hekmatdoost6