ABSTRACT
Type 2 diabetes mellitus (T2DM) is recognized as one of the most prevalent metabolic diseases, and it is mostly associated with oxidative stress, atherosclerosis and dyslipidemia. Paraoxonase 2 (PON2) due to its antioxidant properties may play a role in the atherosclerosis development. Although long-chain omega-3 polyunsaturated fatty acids, such as eicosapentaenoic acid (EPA) have been shown to reduce the risk of cardiovascular disease, the exact mechanism of action is still unknown. Our goal in this study was to determine the effect of EPA administration on gene expression of PON2 in patients with T2DM. Present study was a randomized, controlled double-blind trial. Thirty-six patients with T2DM were randomly allocated to receive 2 g/day EPA (n = 18) or placebo (n = 18) for 8 weeks. There were no significant differences between 2 groups concerning demographic or biochemical variables, and dietary intakes as well (p > 0.05). However, patients received EPA showed a significant increase in the gene expression of PON2 compared with placebo group (p = 0.027). In addition, high-density lipoprotein cholesterol increased and fasting blood sugar decreased significantly after EPA supplementation compared with control group. Taken together, supplementation with 2 g/day EPA could be atheroprotective via the upregulation of PON2 in patients with T2DM.
-
Trial Registration
-
Keywords: Eicosapentaenoic acid; Paraoxonase-2; Gene expression; Type 2 diabetes mellitus; Randomized Controlled Trial
INTRODUCTION
Diabetes is one of the most prevalent endocrine disorders resulting from defect in the insulin resistance and/or insulin secretion [
1]. Type 2 diabetes mellitus (T2DM) is recognized as one of the most common metabolic disorders in developed and developing countries [
2]. Previous reports has anticipated that global prevalence of T2DM will increase to 366 million in 2030 [
3]. It is anticipated that diabetes will devote between 7 to 13 percent of the healthcare budget of worldwide [
4]. T2DM lead to many chronic end-organ damage in the eyes, kidneys, and the brain and other complications including cardiovascular diseases (CVDs) [
5,
6].
The paraoxonase (PON) multigene family contains 3 different members [
7]. PON2 is the first member of the PON family which discovered [
8]. PON2 is nearly expressed in all human tissues, including placenta, heart, liver, kidney, stomach, and small intestine [
9]. In addition, PON2 mRNA is also found in the cells of the artery wall including endothelial cells, and smooth muscle cells [
10]. PON2 protein is mostly located in the cell membrane. Therefore, its mechanism of action is likely related to its location in the cell [
11]. In addition, PONs decrease lipoprotein peroxides like oxidized-low-density lipoprotein (LDL), impede macrophage cholesterol biosynthesis and postpone atherosclerosis [
12]. Because of its antioxidant properties exactly in cellular level; the preventing role of PON2 against development of atherosclerosis could be contributed to high lactonase activity [
12,
13].
Long-chain omega-3 polyunsaturated fatty acids (LC: n-3 PUFA) have antioxidant [
14], anti-inflammatory [
15], antithrombogenic [
16], and antiarteriosclerotic [
17] properties. LC: n-3 PUFA e.g. eicosapentaenoic acid (EPA) found at the great amounts in the fish oil [
18]. Previous report indicated that LC: n-3 PUFA supplementation increase the large high-density lipoprotein (HDL) particles and decrease very-low-density lipoprotein particle size and concentration [
19]. LC: n-3 PUFAs are beneficial against T2DM because of insulin-sensitizing effects and reducing
de novo lipogenesis and hepatic fat accumulation [
20].
Although previous studies investigate the mechanism of EPA effect on gene expression in T2DM like selenoprotein P gene in vitro [
20] and PPARγ and CD36 in clinical trial studies [
21] but to best of our knowledge there is no trial which examine EPA effect on PON2 expression in a randomized controlled trial. So, the present study was aimed to determine the effect of EPA on the gene expression of PON2 in peripheral blood mononuclear cells (PBMCs) of the patients with T2DM.
MATERIALS AND METHODS
Patients and study design
Present study was a randomized double-blind, controlled, trial. Patients were selected from Iran Diabetes Association, Tehran, Iran. In keeping with allocation concealment participants randomly divided in two groups to receive 2 g/day of the softgels of EPA (n = 18) (supplied as 1-g softgels) or placebo (n = 18) for 8 weeks. Both EPA and placebo supplied from same company which were same in form and size as well (EPA softgels containing 75% ethyl ester EPA and placebo containing edible paraffin, Mino Pharmaceutical Co., Tehran, Iran). Due to scarcity of evidence about suitable dosage for EPA, we used the suggested doses in keeping with the past study [
22]. All patients and researchers were uninformed about randomized allocation until the last analysis. They were strictly advised to maintain their nutritional habits and medication dose(s) during intervention in both intervention and control group. Compliance to the intervention controlled
via weekly phone calls and returning the supplement containers, monthly.
Patients were enrolled in the present study if they met following inclusion criteria: At least 1 year after DM diagnosis, (American Diabetes Association [
23]), 25 ≤ body mass index (BMI) < 30 kg/m
2, age between 35–50 years old, taking the antidiabetic's drug(s) dose at least for 3 months. All of inclusion criteria must be met before any subject enrolled to present study. Patients excluded because of following exclusion criteria history of hypertension, arrhythmia, hepatic, renal, gastrointestinal, disease, need to take insulin and compliance below 90%. When at least one of exclusion criteria met, subject excluded from present trial. Patients who take insulin excluded due to poor diabetes and glycemic control. Present study was approved by Research Ethical Committee Center of Tehran University of Medical Sciences (code: 84153) and performed in keeping with Declaration of Helsinki. Written informed consent were obtained from all patients before enrolment to the present trial. The study previously registered in the Clinical Trials (
www.clinicaltrials.gov) (
NCT03258840).
Questionnaires, anthropometry, physical activity and nutritional assessments
Body weight and height was assessed without shoes and in a minimal clothing state to the nearest 0.1 kg and 0.1 cm respectively by using available equipment (Seca, Hamburg, Germany). BMI was computed via following formula: BMI = weight(kg)/(height(m))2. Waist circumference was measured at the center of costal margin and the iliac crest by elastic tape measure.
At the start and the end of the study, each participant filled up a questionnaire containing questions concerning age, sex and lifestyle like smoking, and alcohol consumption. Furthermore, other information including medical history were acquired via questionnaire including measurements of blood pressure and heart rate (systolic blood pressure [SBP], diastolic blood pressure [DBP], and mean blood pressure [MBP]), and pulse pressure (PP). Family history of diseases like CVDs or DM were recorded as well. Hypertension assigned based on the International Diabetes Federation guidelines (≥ 130/85 mmHg) [
24]. Physical activity measured thorough quantitative international physical activity questionnaire level before and after study which was expressed as met-min/day.
At the commencement and ending of trial, dietary intakes were estimated by a 24-hour dietary recall for 3 days. The dietary recalls then assessed using Nutritionist IV software (First Databank, San Bruno, CA, USA) adjusted for Iranian foods.
Biochemical assessments
Fasting blood samples (10 mL) were acquired from the antecubital vein and centrifuged at 3,000 rpm for 10 minutes at 4°C to separate serum. All samples reserved at –80°C until analysis.
Fasting blood sugar (FBS) was determined by glucose oxidase phenol 4-aminoantipyrine peroxidase (GOD/PAP) technique. Serum triglyceride concentration were evaluated by the glycerol-3-phosphate oxidase phenol 4-aminoantipyrine peroxidase (GPO/PAP) technique. Serum cholesterol levels were evaluated by the CHOD-PAP method. The immuno-inhibition assay method was used for measurement of high-density lipoprotein cholesterol (HDL-C) level. Pars Azmoon kit was used for all above-mentioned measurements (Pars Azmoon Inc., Tehran, Iran).
Molecular analysis
Isolation of PBMCs
All samples protected against any contamination according to available guidelines [
25]. PBMCs isolated according to following procedures: 1) PBS added to the remaining blood volume to achieve its original volume, 2) mixture added gradually tubes containing Ficoll, 3) centrifugation performed at 2,500 rpm (1,100 × g) for 20 minutes at 25°C, 4) buffy coat band which contain PBMC was aspirated, and washed with addition of PBS 5) centrifuge at 1,600 rpm (450 × g) for 15 minutes at 25°C. 6) Cell analysis by microscope.
RNA isolation and cDNA synthesis
Total RNA was purified from PBMC using RNeasy Mini Kit (Qiagen, Hilden, Germany), and cDNA synthetized using Quanti Tect Reverse Transcription (Qiagen) in keeping with producer's instructions.
Primers design
The sequences of needed primer were designed by Primer 3 online software using Gene Bank of the National Center for Biotechnology Information (
http://www.basic.nwu.edu/biotools/Primer3.html or http://www.cgivo.2) [
26]. The specificity of primers were confirmed in BLAST domain (
http://www.ncbi.nlm.nih.gov/BLAST). Sequence of primers (as outlined in
Table 1), for the PON2 and β-actin genes were synthesized by Bioneer Co. Ltd. (Daejeon, Korea).
Table 1The sequences of primers used for real-time polymerase chain reaction
Table 1
|
Primer |
Sequence (5′ → 3′) |
|
PON2 |
Forward |
TGTAGACCTTCCACACTGCCACCT |
|
Reverse |
TGGTGCAAAGCTGTGGAGTCCTG |
|
β-actin |
Forward |
CCTGGCACCCAGCACAATGAAG |
|
Reverse |
CTAAGTCATAGTCCGCCTAGAAGC |
Real-time PCR
Analysis of the selected genes were done by real-time PCR in keeping with ABI Step One (Applied Biosystems, Foster City, CA, USA). Amplification of PCR performed according to following procedures 40 cycles of 15 seconds at 95°C, followed by 40 seconds at 60°C. The mRNA expression of PON2 gene was standardized by β-actin as housekeeping gene.
The ratio of expression level computed according to Pfaffl method. In that case the threshold cycle (Ct) number of selected gene from the Ct of β-actin subtracted and raising 2 to the power of this difference. The Ct values expressed as the number of PCR cycles when the fluorescent signal of PCR achieve a persistent threshold. The expressions of PON2 reported relative to β-actin expression, and are shown as mean ± standard deviation (SD). All real-time PCR results measurements performed using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA), [
27].
Findings expressed as mean and standard deviation. Comparison of variables performed using Independent t-test between 2 groups and paired t-test for within group analysis. Values of p < 0.05 were considered statistically significant. All data analysis performed via SPSS software (SPSS Inc., Chicago, IL, USA)
RESULTS
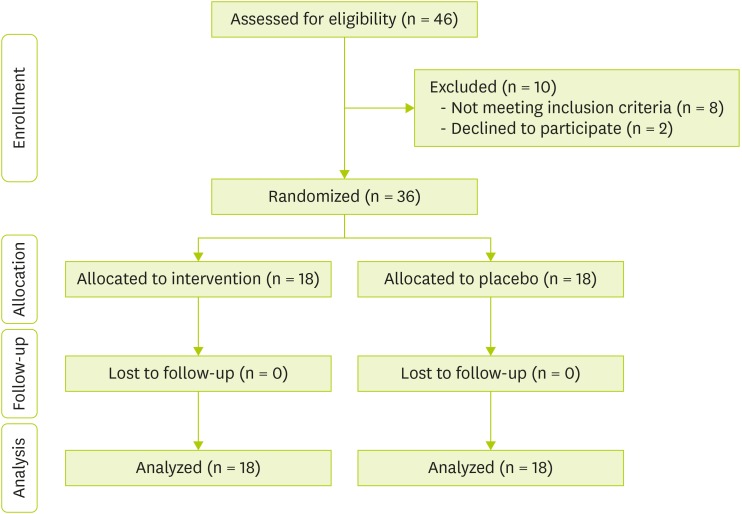
Study population before allocation and after randomization at the end of trial depicted in
Figure 1. Characteristics of the both intervention and control group at the baseline and end of study were shown in
Table 2. There were no statistically significant differences for age, sex, height, weight, BMI, hip circumference, waist/hip ratio, duration of diabetes, physical activity, heart rate, SBP, DBP, and MBP between 2 groups before and after 8 weeks' supplementation (p > 0.05). At the end of trial among biochemical parameters hemoglobin A1C, triglyceride, total cholesterol, and LDL were not different significantly between intervention and placebo group at the beginning and end of trial (
Table 2). There were no significant differences for serum FBS and HDL at the beginning of trial between placebo and intervention group as well. However, EPA supplementation significantly increased HDL and decreased FBS compared with placebo group after 8 weeks' supplementation. In addition, dietary assessment analysis revealed that, there were no statistical significant differences in macronutrients and energy intake between groups (
Table 2).
Figure 1 Patients' flow diagram.
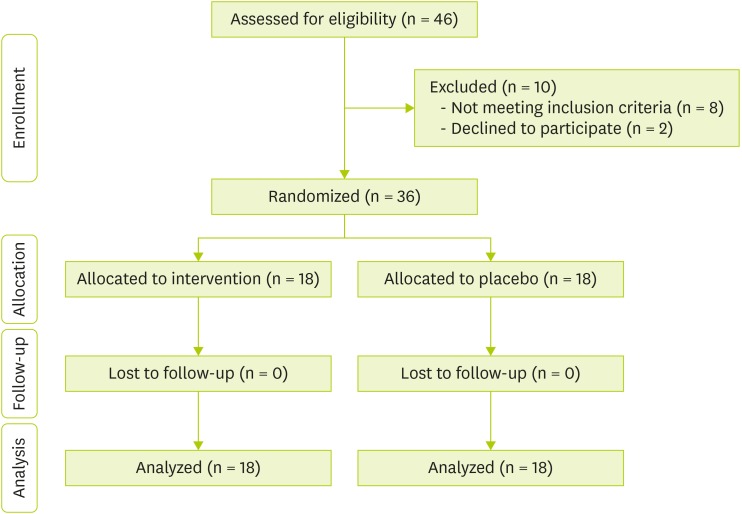
Table 2 Characteristics of the two groups at the baseline and end of study
Table 2
|
Variable |
Baseline |
After 8 weeks' supplementation |
|
Placebo group (n = 18) |
EPA group (n = 18) |
p value |
Placebo group (n = 18) |
EPA group (n = 18) |
p value |
|
No. of patients (female/male) |
9/9 |
9/9 |
- |
- |
- |
- |
|
Age, yr |
44.72 ± 4.69 |
44.44 ± 3.79 |
0.846 |
- |
- |
- |
|
Duration of DM, yr |
6.61 ± 3.68 |
6.44 ± 2.83 |
0.880 |
- |
- |
- |
|
Height, cm |
165.11 ± 8.85 |
165.39 ± 8.12 |
0.922 |
- |
- |
- |
|
Physical activity |
|
|
0.721 |
|
|
0.721 |
|
Low |
3 |
5 |
3 |
5 |
|
Medium |
14 |
12 |
14 |
12 |
|
High |
1 |
1 |
1 |
1 |
|
Weight, kg |
78.30 ± 12.34 |
78.03 ± 12.68 |
0.947 |
78.24 ± 13.39 |
77.15 ± 12.68 |
0.804 |
|
BMI, kg/m2
|
28.92 ± 5.39 |
28.49 ± 3.95 |
0.788 |
28.87 ± 5.61 |
28.17 ± 3.94 |
0.668 |
|
Waist circumference, cm |
97.47 ± 10.93 |
97.55 ± 9.65 |
0.981 |
97.08 ± 11.73 |
96.44 ± 10.16 |
0.862 |
|
Hip circumference, cm |
106.00 ± 11.82 |
105.33 ± 6.69 |
0.836 |
105.61 ± 12.32 |
104.61 ± 7.59 |
0.771 |
|
Waist/hip ratio |
0.92 ± 0.08 |
0.92 ± 0.06 |
0.922 |
0.92 ± 0.07 |
0.92 ± 0.06 |
0.940 |
|
SBP, mmHg |
124.11 ± 15.32 |
124.00 ± 16.25 |
0.983 |
124.89 ± 18.08 |
123.06 ± 18.78 |
0.789 |
|
DBP, mmHg |
80.00 ± 6.69 |
79.78 ± 13.40 |
0.950 |
80.00 ± 7.22 |
79.44 ± 11.83 |
0.876 |
|
MBP, mmHg |
94.70 ± 7.87 |
94.52 ± 13.69 |
0.961 |
94.96 ± 8.98 |
93.98 ± 13.41 |
0.745 |
|
PP, mmHg |
44.11 ± 14.42 |
44.22 ± 9.59 |
0.978 |
44.89 ± 16.83 |
43.62 ± 11.84 |
0.794 |
|
HR, beat/min |
89.44 ± 12.49 |
89.67 ± 10.50 |
0.954 |
89.33 ± 11.73 |
89.33 ± 10.91 |
0.964 |
|
FBS, mg/dL |
138.06 ± 49.13 |
143.72 ± 53.53 |
0.743 |
157.06 ± 52.34 |
121.94 ± 23.56 |
0.016*
|
|
HbA1C, % |
7.47 ± 1.67 |
7.89 ± 1.75 |
0.459 |
7.77 ± 1.42 |
7.86 ± 1.58 |
0.792 |
|
TC, mg/dL |
204.44 ± 43.91 |
211.22 ± 43.57 |
0.645 |
224.28 ± 74.09 |
227.39 ± 70.69 |
0.898 |
|
TG, mg/dL |
221.50 ± 121.49 |
218.61 ± 94.52 |
0.937 |
228.28 ± 165.56 |
180.78 ± 70.54 |
0.271 |
|
LDL-C, mg/dL |
92.61 ± 35.92 |
96.33 ± 38.13 |
0.765 |
95.73 ± 34.66 |
80.11 ± 23.03 |
0.127 |
|
HDL-C, mg/dL |
76.22 ± 32.85 |
77.72 ± 14.92 |
0.861 |
76.50 ± 20.81 |
93.06 ± 25.53 |
0.041*
|
|
Total energy intake, kcal |
2,070.67 ± 307.27 |
1,953.94 ± 297.12 |
0.254 |
1,961.56 ± 232.21 |
1,973.61 ± 274.36 |
0.325 |
|
Carbohydrates intake, g/d |
270.63 ± 50.37 |
260.32 ± 35.44 |
0.483 |
260.32 ± 35.44 |
260.82 ± 42.89 |
0.756 |
|
Proteins intake, g/d |
68.95 ± 20.26 |
63.19 ± 14.78 |
0.337 |
70.09 ± 11.97 |
63.92 ± 14.06 |
0.177 |
|
Lipids intake, g/d |
92.71 ± 19.85 |
86.11 ± 22.68 |
0.359 |
76.39 ± 16.56 |
76.86 ± 20.13 |
0.543 |
|
Fibers intake, g/1,000 kcal |
16.66 ± 4.99 |
14.75 ± 4.64 |
0.243 |
14.64 ± 2.28 |
15.36 ± 4.64 |
0.179 |
Table 3 represents the mRNA expression of PON2 before and after intervention with EPA or placebo. The results showed that the supplementation with EPA significantly increased the gene expression of PON2 compared with control group (p = 0.027,
Table 3).
Table 3 The mRNA expression of PON2 at baseline and after of the supplementation with EPA or placebo
Table 3
|
Group |
EPA |
Placebo |
p value†
|
|
Baseline |
5.31 ± 4.90 |
6.09 ± 5.57 |
|
|
After |
8.43 ± 4.33 |
6.26 ± 4.01 |
0.038 |
|
p value*
|
0.041 |
0.926 |
|
|
Change |
3.12 ± 2.69 |
0.17 ± 4.60 |
0.027 |
DISCUSSION
Oxidative stress is involved in pathogenesis of chronic diseases like diabetes mellitus and atherosclerosis [
28]. Lipid lowering medication and supplements can change the products of lipid peroxidation such as malondialdehyde (MDA) [
28]. In that case, positive effects of fish oil have been reported in diseases which oxidative stress plays a critical role [
29]. Main components of fish oil (EPA and docosahexaenoic acid) and specially EPA have beneficial effects on the levels of serum lipids, and arteriosclerosis [
30], increasing the plasma levels of HDL-C and HDL2-C [
31,
32]. Other beneficial effects of EPA including increasing the production of nitric oxide and anti-inflammatory atheroprotective and antithrombotic properties [
33,
34,
35,
36]. These findings indicate that the EPA consumption may prevent cardiovascular complications in patients with T2DM.
In recent years due to antioxidant and anti-inflammatory role of PON family their role in the field of atherosclerosis have been discussed [
37]. PON2 recognized as a protective protein against the cellular oxidative stress [
10,
38]. It can protect macrophages from triglyceride accumulation [
39] and may prevent the lipid peroxidation thorough hydrolysis of oxidized lipids [
40]. As a mechanism to compensate against the enhancement in oxidative stress, PON2 gene expression and activity will increase [
10,
41] so decreases the release of superoxide anions from the inner membrane of mitochondrion [
42]. PON2 gene expression and its lactonase activity increase in the oxidative stress [
10,
38]. Augmentation in the levels of human PON2 can effectively prevent against the development of atherosclerosis [
43]. The PON2 gene polymorphisms reported to have a crucial metabolic role in vascular disease in a variety of human disorders, such as T2DM [
44], CVD [
44,
45] and early microvascular complications in type 1 DM [
46].
Reduced PON activity has been indicated in a variety of diseases involving oxidative stress including chronic renal failure [
47] and specially in patients with diabetes or CVDs [
48]. There are possible mechanisms for reduced PON activity in diabetes: 1) protein glycation and advanced glycation ends which is the major route of free radical production in patients with diabetes; 2) reduced PON gene expression; 3) inhibition of HDL synthesis or secretion which have been indicated to be related with serum PON [
49].
The present trial shows that EPA supplementation for 8 weeks in T2DM subjects significantly increase the gene expression of PON2 in comparison with control group. Interestingly HDL serum level significantly increased after EPA supplementation compared with control group. The other double-blind, placebo-controlled study in familial hypercholesterolaemia showed that the w-3 supplement in high-risk FCHL subjects result in significant increase of plasma PON concentration [
50]. In other study, fish oil improves serum level of PON1 activity in subjects with rheumatoid arthritis [
28]. Also in that study MDA decreased significantly in fish oil group and serum levels of MDA was inversely related to serum activity of PON1 [
51]. There are several possible mechanisms that have been recommended in previous studies. The n-3 PUFA have been reported to increase PON activity with up-regulated apo A-I. Significant correlation between PON concentration, plasma HDL and apo A-I levels have been reported. Apo A-I stabilize PON2 activity and PON has been identified to be associated with apo A-I–containing HDL particles [
50,
52]. Other pathway could be through increasing the plasma content of the HDL2 subfraction which is more potent in releasing PON by cells [
50].
There were several limitations for our study. First, a relatively small sample size of patients. Second, the mechanism by which EPA influences the gene expression of PON2 has not been clarified and further work is needed to delineate the molecular mechanism of action of EPA on the regulation of PON2. Therefore, further studies to elucidate the general applicability of our result are warranted.
CONCLUSION
The present study provides evidence for the upregulation of PON2 at the gene level by EPA in the patients of T2DM supplemented with EPA. Since the level of the gene expression of PON2 has a significant impact on serum lactonase and PON activities. Thus, it is very beneficial in the reduction of oxidative stress, and as a key regulator in the pathogenesis of atherosclerosis leading to several CVDs. Thereby, the clinical efficiency of EPA in this application has worth evaluating.
Tehran University of Medical Sciences and Health Serviceshttps://doi.org/10.13039/50110000448415202
NOTES
-
Funding: This study was supported by a vice-chancellor of Tehran University of Medical Sciences and by a grant from the Research Deputy of Tehran University of Medical Sciences (project number 15202).
-
Conflict of Interest: The authors declare that they have no competing interests.
ACKNOWLEDGEMENTS
We thank from the staff of Iran Diabetes Association for helping in recruiting of the patients, and from several colleagues from School of Nutrition Sciences, the Tehran University of Medical Sciences for their technical assistance. Authors gratefully acknowledge the Research Council of Tehran University of Medical Sciences for support.
REFERENCES
- 1. Blair M. Diabetes mellitus review. Urol Nurs 2016;36:27-36.
- 2. Chang YC, Chuang LM. The role of oxidative stress in the pathogenesis of type 2 diabetes: from molecular mechanism to clinical implication. Am J Transl Res 2010;2:316-331.
- 3. Rathmann W, Giani G. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:2568-2569.
- 4. International Diabetes Federation. Diabetes atlas. 3rd ed. Brussels: International Diabetes Federation; 2006.
- 5. Chan GC, Tang SC. Diabetic nephropathy: landmark clinical trials and tribulations. Nephrol Dial Transplant 2016;31:359-368.
- 6. Ahangarpour A, Heidari H, Junghani MS, Absari R, Khoogar M, Ghaedi E. Effects of hydroalcoholic extract of Rhus coriaria seed on glucose and insulin related biomarkers, lipid profile, and hepatic enzymes in nicotinamide-streptozotocin-induced type II diabetic male mice. Res Pharm Sci 2017;12:416-424.
- 7. Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, Fu X, Shao M, Brennan DM, Ellis SG, Brennan ML, Allayee H, Lusis AJ, Hazen SL. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA 2008;299:1265-1276.
- 8. Draganov DI, La Du BN. Pharmacogenetics of paraoxonases: a brief review. Naunyn Schmiedebergs Arch Pharmacol 2004;369:78-88.
- 9. Ng CJ, Shih DM, Hama SY, Villa N, Navab M, Reddy ST. The paraoxonase gene family and atherosclerosis. Free Radic Biol Med 2005;38:153-163.
- 10. Rosenblat M, Draganov D, Watson CE, Bisgaier CL, La Du BN, Aviram M. Mouse macrophage paraoxonase 2 activity is increased whereas cellular paraoxonase 3 activity is decreased under oxidative stress. Arterioscler Thromb Vasc Biol 2003;23:468-474.
- 11. Levy E, Trudel K, Bendayan M, Seidman E, Delvin E, Elchebly M, Lavoie JC, Precourt LP, Amre D, Sinnett D. Biological role, protein expression, subcellular localization, and oxidative stress response of paraoxonase 2 in the intestine of humans and rats. Am J Physiol Gastrointest Liver Physiol 2007;293:G1252-G1261.
- 12. Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res 2005;46:1239-1247.
- 13. Précourt LP, Amre D, Denis MC, Lavoie JC, Delvin E, Seidman E, Levy E. The three-gene paraoxonase family: physiologic roles, actions and regulation. Atherosclerosis 2011;214:20-36.
- 14. Kesavulu MM, Kameswararao B, Apparao C, Kumar EG, Harinarayan CV. Effect of omega-3 fatty acids on lipid peroxidation and antioxidant enzyme status in type 2 diabetic patients. Diabetes Metab 2002;28:20-26.
- 15. Figueras M, Olivan M, Busquets S, López-Soriano FJ, Argilés JM. Effects of eicosapentaenoic acid (EPA) treatment on insulin sensitivity in an animal model of diabetes: improvement of the inflammatory status. Obesity (Silver Spring) 2011;19:362-369.
- 16. Terano T, Hirai A, Hamazaki T, Kobayashi S, Fujita T, Tamura Y, Kumagai A. Effect of oral administration of highly purified eicosapentaenoic acid on platelet function, blood viscosity and red cell deformability in healthy human subjects. Atherosclerosis 1983;46:321-331.
- 17. Dyerberg J, Bang HO, Stoffersen E, Moncada S, Vane JR. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet 1978;2:117-119.
- 18. Hagiwara S, Makita Y, Gu L, Tanimoto M, Zhang M, Nakamura S, Kaneko S, Itoh T, Gohda T, Horikoshi S, Tomino Y. Eicosapentaenoic acid ameliorates diabetic nephropathy of type 2 diabetic KKAy/Ta mice: involvement of MCP-1 suppression and decreased ERK1/2 and p38 phosphorylation. Nephrol Dial Transplant 2006;21:605-615.
- 19. Dias CB, Amigo N, Wood LG, Correig X, Garg ML. Effect of diets rich in either saturated fat or n-6 polyunsaturated fatty acids and supplemented with long-chain n-3 polyunsaturated fatty acids on plasma lipoprotein profiles. Eur J Clin Nutr 2017;71:1297-1302.
- 20. Tajima-Shirasaki N, Ishii KA, Takayama H, Shirasaki T, Iwama H, Chikamoto K, Saito Y, Iwasaki Y, Teraguchi A, Lan F, Kikuchi A, Takeshita Y, Murao K, Matsugo S, Kaneko S, Misu H, Takamura T. Eicosapentaenoic acid down-regulates expression of the selenoprotein P gene by inhibiting SREBP-1c protein independently of the AMP-activated protein kinase pathway in H4IIEC3 hepatocytes. J Biol Chem 2017;292:10791-10800.
- 21. Mansoori A, Sotoudeh G, Djalali M, Eshraghian MR, Keramatipour M, Nasli-Esfahani E, Shidfar F, Alvandi E, Toupchian O, Koohdani F. Effect of DHA-rich fish oil on PPARγ target genes related to lipid metabolism in type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. J Clin Lipidol 2015;9:770-777.
- 22. Peet M, Horrobin DF. E-E Multicentre Study Group. A dose-ranging exploratory study of the effects of ethyl-eicosapentaenoate in patients with persistent schizophrenic symptoms. J Psychiatr Res 2002;36:7-18.
- 23. Summary of revisions for the 2010 Clinical Practice Recommendations. Diabetes Care 2010;33(Suppl 1):S3.
- 24. Alberti KG, Zimmet P, Shaw J. International Diabetes Federation: a consensus on type 2 diabetes prevention. Diabet Med 2007;24:451-463.
- 25. Kwok S, Higuchi R. Avoiding false positives with PCR. Nature 1989;339:237-238.
- 26. Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. In Misener S, Krawetz SA, eds, ddBioinformatics methods and protocols. New York (NY): Springer/Humana Press; 1999, pp 365-386.
- 27. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001;29:e45.
- 28. Ghorbanihaghjo A, Kolahi S, Seifirad S, Rashtchizadeh N, Argani H, Hajialilo M, Khabazi A, Alizadeh S, Bahreini E. Effect of fish oil supplements on serum paraoxonase activity in female patients with rheumatoid arthritis: a double-blind randomized controlled trial. Arch Iran Med 2012;15:549-552.
- 29. Miljkovic M, Djuricic I, Kotur-Stevuljevic J, Sobajic S, Spasojevic-Kalimanovska V, Jelic-Ivanovic Z, Kerkez M, Djordjevic V, Djurasic L, Spasic S. Omega-3 fatty acids supplementation effects on paraoxonase-1 enzymatic activity. J Food Nutr Res 2015;54:314-322.
- 30. Singer P, Jaeger W, Wirth M, Voigt S, Naumann E, Zimontkowski S, Hajdu I, Goedicke W. Lipid and blood pressure-lowering effect of mackerel diet in man. Atherosclerosis 1983;49:99-108.
- 31. Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr 2002;76:1007-1015.
- 32. Luo J, Rizkalla SW, Vidal H, Oppert JM, Colas C, Boussairi A, Guerre-Millo M, Chapuis AS, Chevalier A, Durand G, Slama G. Moderate intake of n-3 fatty acids for 2 months has no detrimental effect on glucose metabolism and could ameliorate the lipid profile in type 2 diabetic men. Results of a controlled study. Diabetes Care 1998;21:717-724.
- 33. Okuda Y, Kawashima K, Sawada T, Tsurumaru K, Asano M, Suzuki S, Soma M, Nakajima T, Yamashita K. Eicosapentaenoic acid enhances nitric oxide production by cultured human endothelial cells. Biochem Biophys Res Commun 1997;232:487-491.
- 34. Okumura T, Fujioka Y, Morimoto S, Tsuboi S, Masai M, Tsujino T, Ohyanagi M, Iwasaki T. Eicosapentaenoic acid improves endothelial function in hypertriglyceridemic subjects despite increased lipid oxidizability. Am J Med Sci 2002;324:247-253.
- 35. Tamura Y, Hirai A, Terano T, Takenaga M, Saitoh H, Tahara K, Yoshida S. Clinical and epidemiological studies of eicosapentaenoic acid (EPA) in Japan. Prog Lipid Res 1986;25:461-466.
- 36. Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, Shearman CP, Gallagher PJ, Calder PC, Grimble RF. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet 2003;361:477-485.
- 37. Motti C, Dessì M, Gnasso A, Irace C, Indigeno P, Angelucci CB, Bernardini S, Fucci G, Federici G, Cortese C. A multiplex PCR-based DNA assay for the detection of paraoxonase gene cluster polymorphisms. Atherosclerosis 2001;158:35-40.
- 38. Ng CJ, Wadleigh DJ, Gangopadhyay A, Hama S, Grijalva VR, Navab M, Fogelman AM, Reddy ST. Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. J Biol Chem 2001;276:44444-44449.
- 39. Rosenblat M, Coleman R, Reddy ST, Aviram M. Paraoxonase 2 attenuates macrophage triglyceride accumulation via inhibition of diacylglycerol acyltransferase 1. J Lipid Res 2009;50:870-879.
- 40. Fuhrman B, Volkova N, Aviram M. Oxidative stress increases the expression of the CD36 scavenger receptor and the cellular uptake of oxidized low-density lipoprotein in macrophages from atherosclerotic mice: protective role of antioxidants and of paraoxonase. Atherosclerosis 2002;161:307-316.
- 41. Shiner M, Fuhrman B, Aviram M. Paraoxonase 2 (PON2) expression is upregulated via a reduced-nicotinamide-adenine-dinucleotide-phosphate (NADPH)-oxidase-dependent mechanism during monocytes differentiation into macrophages. Free Radic Biol Med 2004;37:2052-2063.
- 42. Altenhöfer S, Witte I, Teiber JF, Wilgenbus P, Pautz A, Li H, Daiber A, Witan H, Clement AM, Förstermann U, Horke S. One enzyme, two functions: PON2 prevents mitochondrial superoxide formation and apoptosis independent from its lactonase activity. J Biol Chem 2010;285:24398-24403.
- 43. Ng CJ, Hama SY, Bourquard N, Navab M, Reddy ST. Adenovirus mediated expression of human paraoxonase 2 protects against the development of atherosclerosis in apolipoprotein E-deficient mice. Mol Genet Metab 2006;89:368-373.
- 44. Mackness B, McElduff P, Mackness MI. The paraoxonase-2-310 polymorphism is associated with the presence of microvascular complications in diabetes mellitus. J Intern Med 2005;258:363-368.
- 45. Leus FR, Zwart M, Kastelein JJ, Voorbij HA. PON2 gene variants are associated with clinical manifestations of cardiovascular disease in familial hypercholesterolemia patients. Atherosclerosis 2001;154:641-649.
- 46. Kao Y, Donaghue KC, Chan A, Bennetts BH, Knight J, Silink M. Paraoxonase gene cluster is a genetic marker for early microvascular complications in type 1 diabetes. Diabet Med 2002;19:212-215.
- 47. Rasic-Milutinovic Z, Popovic T, Perunicic-Pekovic G, Arsic A, Borozan S, Glibetic M. Lower serum paraoxonase-1 activity is related to linoleic and docosahexanoic fatty acids in type 2 diabetic patients. Arch Med Res 2012;43:75-82.
- 48. Haraguchi Y, Toh R, Hasokawa M, Nakajima H, Honjo T, Otsui K, Mori K, Miyamoto-Sasaki M, Shinohara M, Nishimura K, Ishida T, Hirata K. Serum myeloperoxidase/paraoxonase 1 ratio as potential indicator of dysfunctional high-density lipoprotein and risk stratification in coronary artery disease. Atherosclerosis 2014;234:288-294.
- 49. Zarei M, Fakher S, Tabei SM, Javanbakht MH, Derakhshanian H, Farahbakhsh-Farsi P, Sadeghi MR, Mostafavi E, Djalali M. Effects of vitamin A, C and E, or omega-3 fatty acid supplementation on the level of paraoxonase and arylesterase activity in streptozotocin-induced diabetic rats: an investigation of activities in plasma, and heart and liver homogenates. Singapore Med J 2016;57:153-156.
- 50. Calabresi L, Villa B, Canavesi M, Sirtori CR, James RW, Bernini F, Franceschini G. An ω-3 polyunsaturated fatty acid concentrate increases plasma high-density lipoprotein 2 cholesterol and paraoxonase levels in patients with familial combined hyperlipidemia. Metabolism 2004;53:153-158.
- 51. Baskol G, Demir H, Baskol M, Kilic E, Ates F, Kocer D, Muhtaroglu S. Assessment of paraoxonase 1 activity and malondialdehyde levels in patients with rheumatoid arthritis. Clin Biochem 2005;38:951-955.
- 52. Burillo E, Mateo-Gallego R, Cenarro A, Fiddyment S, Bea AM, Jorge I, Vázquez J, Civeira F. Beneficial effects of omega-3 fatty acids in the proteome of high-density lipoprotein proteome. Lipids Health Dis 2012;11:116.
 , Mohammad Hassan Javanbakht1
, Mohammad Hassan Javanbakht1 , Ehsan Ghaedi1
, Ehsan Ghaedi1 , Hamed Mohammadi2
, Hamed Mohammadi2 , Mahmoud Djalali1
, Mahmoud Djalali1