ABSTRACT
Numerous clinical trials have examined the beneficial effects of Juglans regia leaf extract (JRLE) in patients with type 2 diabetes mellitus (T2DM); however, the results of these studies are inconsistent. Therefore, we conducted the current systematic review and meta-analysis to evaluate the effect of JRLE on glycemic control and lipid profile in T2DM patients. We searched online databases including PubMed, Scopus, EMBASE, and Web of Science for randomized controlled clinical trials that examined the effect of JRLE on glycemic and lipid indices in T2DM patients. Data were pooled using both fixed and random-effect models and weighted mean difference (WMD) was considered as the overall effect size. Of the total records, 4 eligible studies, with a total sample size of 195 subjects, were included. The meta-analysis revealed that JRLE supplementation significantly reduces fasting blood glucose (WMD, −18.04; 95% confidence interval [CI], −32.88 mg/dL, −3.21 mg/dL; p = 0.017) and significantly increases fasting insulin level (WMD, 1.93; 95% CI, 0.40 U/L, 3.45 U/L; p = 0.014). Although the overall effect of JRLE supplementation on hemoglobin A1c was not significant, a significant reduction was seen in studies with an intervention duration of > 8 weeks (WMD, −0.64; 95% CI, −1.16%, −0.11%; p = 0.018). Moreover, we also found no significant change in lipid parameters. Our findings revealed a beneficial effect of JRLE supplementation on glycemic indices in T2DM patients, but no significant improvement was found for lipid profile parameters.
-
Keywords: Juglans; Glycemic control; cholesterol; Diabetes mellitus; HDL-cholesterol; LDL-cholesterol
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is one of the most prevalent metabolic disorders globally, where its’ incidence is predicted to reach 366 million cases by 2030 [
1,
2]. The main characteristics of the disease are hyperinsulinemia, insulin resistance, and β-cells decline [
3], concomitant to dyslipidemia [
4]. The latter includes abnormalities in concentrations of triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), or total cholesterol (TC), which are major risk factors for cardiovascular diseases (CVDs) [
4,
5]. Given the adverse effects of T2DM on vital organs function, such as the kidney [
6], liver, gastrointestinal system [
3], and nervous system [
7], the management of T2DM is pivotal, and mainly achieved via exercise and dietary modification [
8,
9]. In recent decades, the use of alternative medicine, especially herbs, to treat different diseases such as diabetes and CVDs, has grown in popularity, worldwide [
10,
11,
12].
The leaves of
Juglans regia (walnut) have been used in traditional medicines for their apparent antimicrobial, anthelmintic, keratolytic, and antidiarrheal properties, and are rich in polyphenolic compounds and flavonoids [
13]. Dietary flavonoids and polyphenols, which can be found in many foods and medicinal plants, are considered to be responsible for the reported health benefits of the
J. regia L. leaves and play an important role in the prevention and treatment of several chronic diseases, such as diabetes and CVDs caused by oxidative stress [
14]. The constituents in walnut leaf have shown antioxidant, anti-inflammatory, and anticancer effects, mainly via free radicals scavenging [
15,
16]. Glucose and lipid-lowering effects following
J. regia leaf extract (JRLE) administration have been reported and posited as a clinically promising therapy in diabetic rats [
17,
18]. These beneficial influences are mainly attributed to improving β-cells responsiveness and insulin secretion [
17,
19,
20]. Due to its promising results in T2DM patients, it seems that JRLE can be used alone, or in combination with other herbs to improve therapeutic influences on glycemic and/or lipid profiles in T2DM [
21].
However, current evidence from trial studies investigating the effectiveness of JRLE on blood glucose and lipid parameters of T2DM patients in humans is controversial [
13,
22,
23,
24]. Rabiei et al. [
13] didn’t observe any significant effects of 200 mg/d JRLE on blood glucose and homeostasis model assessment of insulin resistance (HOMA-IR) levels whilst Hosseini et al. [
23] reported that consumption of 100 mg JRLE 2 times a day improves glycemic control and lipid profile. To the best of our knowledge, no systematic review has evaluated the effects of JRLE, in the management of metabolic parameters in T2DM patients. Therefore, the results of this study can potentially help to select appropriate treatment options for healthy people and chronic patients. Accordingly, this study aimed to conduct a systematic review and meta-analysis to evaluate the effect of JRLE on glycemic control and lipid profile in T2DM patients.
MATERIALS AND METHODS
This study was designed and conducted according to the guidelines of the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement.
Search strategy and data collection
Searching was performed up to 15 November 2020 in the following databases: PubMed, Scopus, EMBASE, and Web of Science. No restrictions on language were considered. The search terms utilized are mentioned below:
("juglans"[MeSH] OR walnuts[MeSH] OR walnut OR "walnut leaf" OR "juglans regia leaf" OR "walnut leaf" OR "juglans regia leaf") AND (metabolic OR "Metabolic Syndrome"[Mesh] OR "mets" OR "blood glucose" OR "plasma glucose" OR "blood sugar" OR "plasma sugar" OR "FPG" OR "FPS" OR "FBG" OR "FBS" OR glucose OR "Glycated Hemoglobin A"[Mesh] OR "hba1c" OR "glycosylated hemoglobin" OR "glycated hemoglobin" OR insulin OR diabetic OR diabetes OR "Diabetes Mellitus"[Mesh] OR "t2dm" OR "type 2 diabetes" OR "non-insulin dependent") OR ("Cholesterol"[MeSH] OR "low-density lipoprotein" OR LDL OR LDL-C OR LDL-cholesterol OR "high-density lipoprotein" OR "HDL" OR "HDL-C" OR "HDL-cholesterol" OR triglyceride OR hyperlipidemia OR "Hyperlipidemias"[Mesh] OR hyperlipidemic OR dyslipidemia OR dyslipidemic OR lipoprotein) OR ("Liver enzyme" OR "Liver Function Test" OR "Alanine Transaminase"[MeSH] OR "Aspartate Aminotransferases"[Mesh] OR "Alkaline Phosphatase"[Mesh] OR "gamma-Glutamyltransferase"[Mesh] OR "Glutamic Alanine Transaminase" OR "GlutamicAlanine Transaminase" OR "Alanine Aminotransferase" OR "Aspartate Aminotransferase" OR "Aspartate Transaminase" OR "Glutamic-Oxaloacetic Transaminase" OR "alanine aminotransferase" OR "ALP" OR "SGOT" OR "ALT" OR "AST" OR "SGPT" OR "GGT") AND (administration[tiab] OR intervention[tiab] OR "controlled trial"[tiab] OR randomized[tiab] OR random[tiab] OR Randomly[tiab] OR Placebo[tiab] OR Assignment[tiab] OR "clinical trial"[tiab] OR trial[tiab] OR randomised[tiab] OR "Randomized Controlled Trial"[Publication Type] OR "Controlled Clinical Trial"[Publication Type] OR "Placebos"[Mesh] OR "Placebo Effect"[Mesh] OR "Clinical Trial"[Publication Type] OR "Clinical Trials as Topic"[Mesh]).
Reference lists of relevant published research were also reviewed for potentially relevant studies.
Study selection
The inclusion criteria for this meta-analysis were: (1) randomized clinical trial, in either parallel or crossover design vs. placebo control, (2) adults (≥ 18 years) with T2DM, (3) administration of JRLE in any form (powder, aqueous, ethanolic, etc.), (4) presentation of adequate information on the baseline and at the end of the study in both intervention and control groups. Exclusion criteria were the following: (1) nonclinical studies, (2) uncontrolled trials, (3) lack of sufficient information on baseline or follow-up, and (4) supplementation with an active comparator in the control group.
Data extraction
Data extraction was performed independently by 2 reviewers (MD and AM) and was checked by a third reviewer (ED) for accuracy. The following information was extracted: author’s name, publication year, study design and arms, characteristics of the participants (age, health conditions, and sample size), details of the trial (dose of interventions, duration, form of JRLE), mean and standard deviation (SD) of levels of selected parameters at baseline and the end of the trial. Conversion of units and statistical values of measurements were performed accordingly.
Quality assessment
The Cochrane Risk of Bias (ROB) Assessment tool was used to evaluate each study. The assessment contains the following domains: adequacy of sequence generation, incomplete outcome data adequately addressing, allocation concealment, blinding of participants, selective outcome reporting, and other potential causes of bias. According to the recommendations of the Cochrane Handbook, ROB for each domain was categorized as low, high, or unclear [
25]. ROB of included studies is shown in Supplementary Table 1.
Mean differences and their SDs between the baseline and final value of the factors under study, were obtained from the studies to calculate the effect size. Meta-analysis was performed using Stata, version 11.2 (StataCorp LLC, College Station, TX, USA). The Cochrane’s Q test and the I
2 statistic were used to evaluate statistical heterogeneity across studies. I
2 value
> 50% or
p < 0.05 was considered as significant heterogeneity, and a random-effect model was used [
25]. Subgroup analysis was performed to examine probable sources of heterogeneity, according to predefined variables including dosage (≤ 200 vs. > 200 mg/day) and duration (≤ 8 vs. > 8 weeks). For studies with missing values of SD for mean difference, the following formula was used: SD
= square root ((SD
pretreatment)
2
+ (SD
posttreatment)
2 − (2R
× SD
pretreatment
× SD
posttreatment)), where correlation coefficient (R) was assumed equal to 0.5 [
26]. Effect sizes were expressed as the mean difference and 95% confidence interval (CI). Egger’s linear regression and Begg’s test were used to evaluate the publication bias [
27]. The p values < 0.05 were considered, a priori, to represent statistical significance.
RESULTS
Out of 1,296 articles, and following the removal of duplicates, the titles and abstracts of 685 articles were screened. After reading the titles or abstracts, 576 articles were excluded, as they were unrelated. After assessing the full text of 109 potentially related articles, 4 articles were included in our analysis. The main reasons for exclusion were as follows: 103 articles did not contain walnut (
J. regia) “leaf” as intervention, in one article JRLE was used in combination with other components (or drugs), and one article was published as a letter.
Figure 1 represents the flow diagram of study selection.
Figure 1 Flow diagram of study selection.
Characteristics of the included studies
The characteristics of 4 randomized controlled trials (RCTs) included in the meta-analysis are outlined in
Table 1. The review included 195 individuals from 4 studies. All 4 RCTs were conducted in Iran, on T2DM patients, and both genders [
13,
22,
23,
24]. Sample sizes varied from 37 to 61, the duration of intervention in the studies was 8 and 12 weeks, whilst the dosage of JRLE varied from 200 to 750 mg/day; where in one study, the intervention was defined as 100 mg/day for one week, then continued with 200 mg/day for 7 weeks [
13]. The mean age of participants was between 49.9 and 58.1 years. None of the RCTs had a low risk of bias in all domains of the Cochrane ROB Assessment tool (Supplementary Table 1).
Table 1Characteristics of the included studies
Table 1
|
Author |
Design |
Participants |
Health condition |
Age (yr)*
|
Intervention |
Duration (wk) |
Outcomes (changes)†
|
|
Treatment group |
Control group |
Treatment group |
Control group |
|
Rabiei et al. (2018) [13] |
RA/Parallel |
M/F: 39 |
T2DM |
Int: 50.50 ± 8.30 |
100 mg/day (1 wk) then 200 mg/day (7 wk) hydroalcoholic extract |
Placebo: microcrystalline cellulose |
8 |
FBG: −12.20 ± 44.12 |
FBG: 12.90 ± 59.40 |
|
Int: 20 |
Con: 49.90 ± 8.60 |
PPG: 24.50 ± 85.77 |
PPG: −47.00 ± 62.23 |
|
Con: 19 |
Insulin: 0.90 ± 3.80 |
Insulin: −1.70 ± 3.20 |
|
HBA1c: −0.10 ± 1.57 |
HBA1c: −0.80 ± 1.15 |
|
TG: −9.10 ± 84.07 |
TG: 16.30 ± 94.52 |
|
TC: −7.50 ± 37.45 |
TC: −6.90 ± 33.89 |
|
HDL-C: 2.10 ± 9.20 |
HDL-C: 1.30 ± 12.95 |
|
LDL-C: −9.70 ± 27.36 |
LDL-C: −9.30 ± 19.84 |
|
AST: 0.10 ± 5.48 |
AST: −4.50 ± 8.52 |
|
ALT: −0.50 ± 7.43 |
ALT: −6.40 ± 10.75 |
|
Abdoli et al. (2017) [22] |
RA/DB/Parallel |
M/F: 37 |
T2DM |
Int: 56.60 ± 6.10 |
750 mg/day aqueous extract |
Placebo: toast powder |
12 |
FBG: −17.50 ± 32.20 |
FBG: 3.90 ± 42.00 |
|
Int: 19 |
Con: 55.10 ± 7.90 |
PPG: −48.00 ± 59.7 |
PPG: −13.80 ± 89.10 |
|
Con: 18 |
HBA1c: −0.90 ± 1.20 |
HBA1c: −0.30 ± 1.00 |
|
TG: −10.50 ± 42.40 |
TG: −6.80 ± 37.90 |
|
TC: −12.40 ± 45.00 |
TC: −2.50 ± 38.70 |
|
HDL-C: 4.00 ± 16.80 |
HDL-C: 8.50 ± 11.30 |
|
LDL-C: −15.20 ± 31.10 |
LDL-C: −1.10 ± 26.10 |
|
Hosseini et al. (2014) [23] |
RA/DB/Parallel |
M/F: 61 |
T2DM |
Int: 54.80 ± 1.13 |
200 mg/day leaf powder |
Placebo: NR |
12 |
FBG: −22.00 ± 60.10 |
FBG: −9.69 ± 66.22 |
|
Int: 32 |
Con: 55.40 ± 0.70 |
HBA1c: −0.99 ± 1.49 |
HBA1c: −0.31 ± 1.62 |
|
Con: 29 |
TG: −16.15 ± 90.32 |
TG: 21.18 ± 87.03 |
|
TC: −12.47 ± 45.00 |
TC: 5.55 ± 48.49 |
|
HDL-C: −1.28 ± 59.28 |
HDL-C: 0.03 ± 9.15 |
|
LDL-C: −6.95 ± 30.75 |
LDL-C: 2.69 ± 33.68 |
|
AST: −1.09 ± 5.15 |
AST: −1.62 ± 4.36 |
|
ALT: 0.32 ± 3.35 |
ALT: −0.29 ± 2.97 |
|
Hosseini et al. (2014) [24] |
RA/DB/Parallel |
M/F: 58 |
T2DM |
Int: 58.10 ± 4.20 |
400 mg/day aqueous extract |
Placebo: NR |
8 |
FBG: −21.00 ± 60.25 |
FBG: −10.00 ± 66.56 |
|
Int: 30 |
Con: 56.20 ± 7.30 |
Insulin: 1.80 ± 4.47 |
Insulin: 0.50 ± 3.75 |
|
Con: 28 |
HBA1c: −0.90 ± 1.47 |
HBA1c: −0.60 ± 1.60 |
|
AST: 1.00 ± 5.15 |
AST: −0.30 ± 4.35 |
|
ALT: 1.00 ± 3.37 |
ALT: −1.00 ± 2.96 |
Main results from the meta-analysis
Effect of JRLE supplementation on glycemic profile
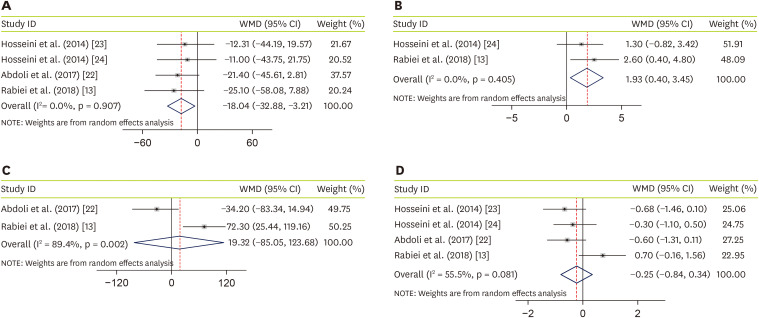
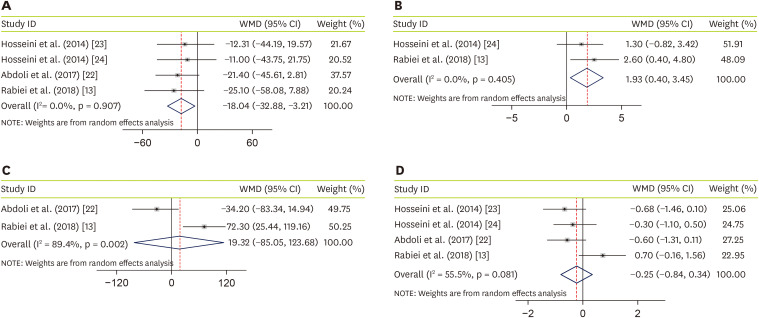
Four studies, with a total sample size of 195 subjects, evaluated the effect of JRLE on fasting blood glucose (FBG) [
13,
22,
23,
24], and indicated that JRLE supplementation yielded a significant reduction in FBG compared with the control group (weighted mean difference [WMD], −18.04; 95% CI, −32.88 mg/dL, −3.21 mg/dL, p = 0.017;
Figure 2A), with no significant heterogeneity between studies (p = 0.91; I
2 = 0.0%). Effectiveness of JRLE supplementation on fasting insulin level were evaluated in 2 studies [
13,
24]. Result of meta-analysis, including 97 participants, showed a significant increase in fasting insulin level (WMD, 1.93; 95% CI, 0.40 U/L, 3.45 U/L; p = 0.014;
Figure 2B), with no considerable between-study heterogeneity (p = 0.4; I
2 = 0.0%). Combining 2 studies that evaluated the effect of JRLE on postprandial glucose (PPG) [
13,
22], including 97 participants, resulted not-significant change in PPG (WMD, 19.32; 95% CI, −85.05 mg/dL, 123.68 mg/dL; p = 0.212;
Figure 2C), while a significant between-study heterogeneity was found (p = 0.002; I
2 = 89.4%). By combining effect sizes from 4 studies [
13,
22,
23,
24], including 195 participants, we found no significant effect of JRLE on hemoglobin A1c (HbA1c), compared to control group (WMD, −0.25; 95% CI, −0.84%, 0.34%; p = 0.41;
Figure 2D), with a considerable between-study heterogeneity (p = 0.081; I
2 = 55.5%).
Figure 2
Forest plot for the effect of J. regia leaf extract on glycemic profiles: (A) fasting blood glucose, (B) insulin level, (C) postprandial glucose, and (D) hemoglobin A1c. Data are expressed as WMDs between intervention and control groups. Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from the fixed-effects analysis.
WMD, weighted mean difference; CI, confidence interval.
Effect of JRLE supplementation on lipid profile
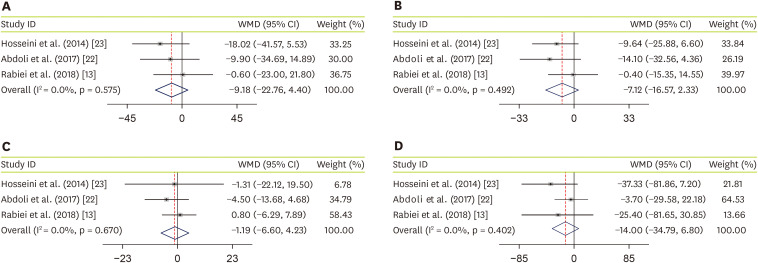
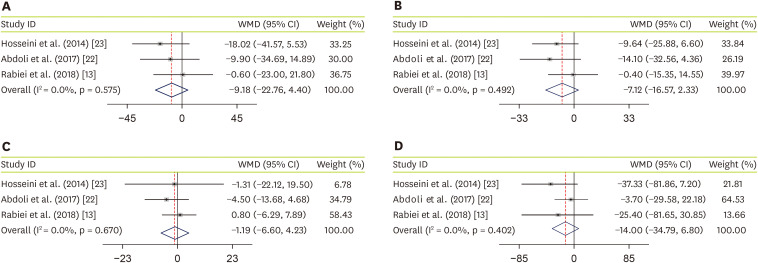
Three studies, with a total sample size of 137 subjects, evaluated the effect of JRLE on TC [
13,
22,
23], and indicated that JRLE supplementation elicited no significant change in TC compared with the control group (WMD, −9.18; 95% CI, −22.76 mg/dL, 4.40 mg/dL; p = 0.185;
Figure 3A), with no significant heterogeneity between studies (p = 0.58; I
2 = 0.0%). The effectiveness of JRLE supplementation on LDL-C was evaluated in 3 studies [
13,
22,
23]. Of the following meta-analysis, including 137 participants, showed no significant change in LDL-C (WMD, −7.12; 95% CI, −16.57 mg/dL, 2.33 mg/dL, p = 0.14;
Figure 3B), with no considerable between-study heterogeneity (p = 0.49; I
2 = 0.0%). Combining effect sizes from 3 studies that evaluated effect of JRLE on HDL-C [
13,
22,
23], including 137 participants, resulted no significant change in HDL-C (WMD, −1.19; 95% CI, −6.60 mg/dL, 4.23 mg/dL; p = 0.67;
Figure 3C), while between-study heterogeneity was not found (p = 0.67; I
2 = 0.0%). Through combining effect sizes from 3 studies [
13,
22,
23], including 137 participants, we found no significant effect of JRLE on TG, compared to control group (WMD, −14.00; 95% CI, −34.79 mg/dL, 6.80 mg/dL; p = 0.19;
Figure 3D), with no considerable between-study heterogeneity (p = 0.40; I
2 = 0.0%).
Figure 3
Forest plot for the effect of J. regia leaf extract on lipid profile: (A) total cholesterol, (B) low-density lipoprotein cholesterol, (C) high-density lipoprotein cholesterol, and (D) triglyceride. Data are expressed as WMDs between intervention and control groups. Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from the fixed-effects analysis.
WMD, weighted mean difference; CI, confidence interval.
Secondary outcomes
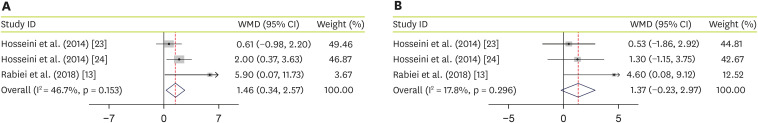
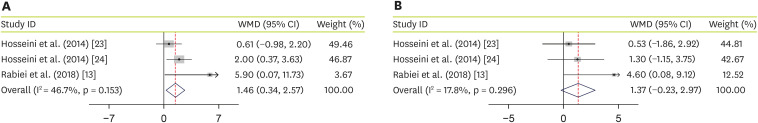
As an additional outcome, effectiveness of JRLE on 2 liver enzymes was evaluated. By pooling data from 3 studies [
13,
23,
24], including 158 participants, we found a significant increase in alanine transaminase (ALT) (WMD, 1.46; 95% CI, 0.34, 2.57; p = 0.01) with no significant heterogeneity between studies (I
2 = 46.7%; p = 0.153) (
Figure 4A). Pooled data from 3 studies [
13,
23,
24], including 158 participants, showed no significant effectiveness of JRLE supplementation on aspartate transaminase (AST) levels (WMD, 1.37; 95% CI, −0.23, 2.97; p = 0.09) with no considerable heterogeneity between studies (I
2 = 17.8%; p = 0.3) (
Figure 4B).
Figure 4
Forest plot for the effect of J. regia leaf extract on liver enzymes: (A) alanine transaminase, (B) aspartate transaminase. Data are expressed as mean differences between intervention and control groups. Horizontal lines represent 95% CIs. Diamonds represent pooled estimates from the fixed-effects analysis.
WMD, weighted mean difference; CI, confidence interval.
Publication bias
The Egger’s test performed to assess publication bias, otherwise, in instances of ≤ 2 effect sizes, Egger’s test was not applicable and Begg’s test was used. No publication bias was found for FBG (p = 0.59), HbA1c (p = 0.187), TC (p = 0.63), LDL-C (p = 0.22), HDL-C (p = 0.81), TG (p = 0.35), ALT (p = 0.4), or AST (p = 0.15), using the Egger’s test. Whilst no publication bias was found for insulin (p = 1.0) or PPG (p = 1.0) using the Begg’s test.
Findings from the subgroup analysis
Table 2 shows the data from subgroup analysis by the dosage of JRLE. Levels of insulin and PPG in the low-dose groups (≤ 200 mg/day) significantly increased (insulin: WMD, 2.60; 95% CI, 0.40 U/L, 4.80 U/L; PPG: WMD, 72.30; 95% CI, 25.44 mg/dL, 119.16 mg/dL), compared to high-dose groups. Non-significant results were found for levels of FBS, HbA1c, TC, LDL-C, HDL-C, and TG, respectively.
Table 2Subgroup analysis by dosage of J. regia leaf extract
Table 2
|
Metabolic parameter |
Dosage (mg/day) |
Effect size |
WMD |
95% CI |
p value |
Heterogeneity |
|
I2 (%) |
p-heterogeneity |
|
FBG (mg/dL) |
≤ 200 |
2 |
−18.49 |
−41.41, 4.43 |
0.110 |
0.00 |
0.58 |
|
> 200 |
2 |
−17.73 |
−37.19, 1.74 |
0.070 |
0.00 |
0.62 |
|
Insulin (U/L) |
≤ 200 |
1 |
2.60 |
0.40, 4.80 |
0.020 |
|
|
|
> 200 |
1 |
1.30 |
−0.82, 3.42 |
0.230 |
|
|
|
PPG (mg/dL) |
≤ 200 |
1 |
72.30 |
25.44, 119.16 |
0.002 |
|
|
|
> 200 |
1 |
−34.20 |
−83.34, 14.94 |
0.170 |
|
|
|
HbA1c (%) |
≤ 200 |
2 |
0.00 |
−1.35, 1.35 |
0.990 |
81.40 |
0.02 |
|
> 200 |
2 |
−0.47 |
−1.00, 0.06 |
0.080 |
0.00 |
0.58 |
|
TC (mg/dL) |
≤ 200 |
2 |
−8.87 |
−25.10, 7.36 |
0.280 |
9.40 |
0.29 |
|
> 200 |
1 |
−9.90 |
−34.69, 14.89 |
0.430 |
|
|
|
LDL-C (mg/dL) |
≤ 200 |
2 |
−4.64 |
−15.64, 6.36 |
0.410 |
0.00 |
0.41 |
|
> 200 |
1 |
−14.10 |
−32.56, 4.36 |
0.130 |
|
|
|
HDL-C (mg/dL) |
≤ 200 |
2 |
0.58 |
−6.13, 7.29 |
0.860 |
0.00 |
0.85 |
|
> 200 |
1 |
−4.50 |
−13.68, 4.68 |
0.330 |
|
|
|
TG (mg/dL) |
≤ 200 |
2 |
−32.73 |
−67.65, 2.18 |
0.060 |
0.00 |
0.74 |
|
> 200 |
1 |
−3.70 |
−29.58, 22.18 |
0.770 |
|
|
In the subgroup analysis by the duration of treatment (
Table 3), we observed significantly increased PPG (WMD, 72.30; 95% CI, 25.44 mg/dL, 119.16 mg/dL) in a shorter duration of treatment (≤ 8 weeks); moreover, the levels of HbA1c in the longer duration (> 8 weeks) were significantly decreased (WMD, −0.64; 95% CI, −1.16%, −0.11%). Non-significant results were found for levels of FBG, TC, LDL-C, HDL-C, and TG, respectively.
Table 3Subgroup analysis by the duration of treatment with J. regia leaf extract
Table 3
|
Metabolic parameter |
Duration (wk) |
Effect size |
WMD |
95% CI |
p value |
Heterogeneity |
|
I2 (%) |
p-heterogeneity |
|
FBG (mg/dL) |
≤ 8 |
2 |
−18.00 |
−41.24, 5.24 |
0.130 |
0.00 |
0.55 |
|
> 8 |
2 |
−18.07 |
−37.35, 1.20 |
0.070 |
0.00 |
0.66 |
|
PPG (mg/dL) |
≤ 8 |
1 |
72.30 |
25.44, 119.16 |
0.002 |
|
|
|
> 8 |
1 |
−34.20 |
−83.34, 14.94 |
0.170 |
|
|
|
HbA1c (%) |
≤ 8 |
2 |
0.19 |
−0.79, 1.17 |
0.710 |
64.20 |
0.09 |
|
> 8 |
2 |
−0.64 |
−1.16, −0.11 |
0.010 |
0.00 |
0.89 |
|
TC (mg/dL) |
≤ 8 |
1 |
−0.60 |
−23.00, 21.8 |
|
|
|
|
> 8 |
2 |
−14.17 |
−31.24, 2.91 |
0.100 |
0.00 |
0.64 |
|
LDL-C (mg/dL) |
≤ 8 |
1 |
−0.40 |
−15.35, 14.55 |
0.960 |
|
|
|
> 8 |
2 |
−11.59 |
−23.78, 0.61 |
0.060 |
0.00 |
0.72 |
|
HDL-C (mg/dL) |
≤ 8 |
1 |
0.80 |
−6.29, 7.89 |
0.820 |
|
|
|
> 8 |
2 |
−3.98 |
−12.38, 4.42 |
0.350 |
0.00 |
0.78 |
|
TG (mg/dL) |
≤ 8 |
1 |
−25.40 |
−81.65, 30.85 |
0.380 |
|
|
|
> 8 |
2 |
−12.19 |
−34.57, 10.18 |
0.290 |
38.90 |
0.20 |
DISCUSSION
We conducted a systematic review to evaluate the effectiveness of JRLE supplementation for improving glucose control and lipid profile in T2DM patients. Accordingly, JRLE supplementation had no significant effect on TC, LDL-C, and HDL-C; however, it significantly reduced FBG and significantly increased ALT (albeit a secondary outcome). To the best of our knowledge, this systematic review is the first to have investigated the association between JRLE supplementation and improving glucose and lipid profile in diabetic patients. Following examination of the extant literature, there is a large inconsistency in the results of published studies, thus highlighting the necessity for further research to confirm the supplemental effects of JRLE on glucose control and lipid profile in T2DM patients. As we mentioned before, a double-blind, placebo-controlled clinical trial in 2018 with 50 diabetic patients, did not report any significant effect of 200 mg/day JRLE consumption on blood glucose level and HOMA-IR score after 8 weeks. In another double-blind clinical trial, 76 diabetic patients received combined capsules as herbal medicine which 20% of that was powder from the walnut leaf. After 12 weeks, levels of fasting, 2-hour PPG, and HbA1c decreased significantly compared to the control group [
21]. Also, another study in 2020 showed that consumption of walnut (
J. regia) in 90 hyperlipidemic patients after 56 days decreased TC, TG, LDL-C, and increased HDL-C, significantly [
28].
Increases in PPG, following JRLE supplementation, were more evident in trials with intervention durations less than 8 weeks. Indeed, although we did not observe any significant effect of JRLE supplementation on serum PPG levels in general, a significant effect on these glucose markers was seen for studies with less than 8 weeks of intervention. Moreover, the levels of HbA1c were significantly decreased only in studies with an intervention duration longer than 8 weeks. Besides study duration, it is evident that JRLE dosages might also play a role; indeed, dosages of less than 200 mg per day appeared more efficacious than higher doses for increasing insulin and PPG.
We found that JRLE had no significant effect on HbA1cwhen administered for short time. It should be noted that JRLE is a rich source of polyunsaturated fatty acid (PUFA); therefore, long-term intake of JRLE might have beneficial effects linked to fat deposition and hormonal regulatory systems. The exact mechanism by which JRLE affects blood glucose and lipids remains unclear, although, in previous studies, the possible mechanisms of JRLE in the regulation of inflammatory markers have been investigated. Oxidative stress and inflammation-related indicators play a vital role in mediating the insulin resistance, impaired insulin secretion, and late diabetic complications [
29,
30,
31]. The supposed mechanisms of JRLE on blood glucose in T2DM were as asserted to be related to the antioxidant components present in JRLE, since inflammation and activated innate immunity are important factors in the pathogenesis of T2DM [
32,
33,
34]. Several putative theories have been proposed for the anti-inflammatory effects of JRLE; for instance, walnut leaves are an excellent source of phenolic acids and flavonoids including 3- and 5-caffeoylquinic acids and quercetins [
35,
36,
37,
38]. Further, some authors have also suggested that lower levels of serum glucose and good control of hyperglycemia may be manifest following antioxidant activity of phenolic compounds, among diabetic patients [
39,
40]. The flavonoids in JRLE also present significant radical scavenging activities [
32]; for instance, in a murine model, high glucose concentrations decreased following JRLE administration [
41].
Based on experimental studies, an anti-diabetic mechanism of JRLE has been suggested to elicit positive effects on absorption of dietary carbohydrates [
42], in addition to potentially stimulating GLUT-4 to promote glucose consumption by peripheral tissues [
43]. Moreover, previous studies have emphasized a significant effect of JRLE on glucose and insulin metabolism, through β-cells, by increasing the release of insulin from stimulated β-cells [
44,
45,
46].
In animal-based studies, significant reductions in liver pyruvate carboxykinase activity and increased liver glycogen phosphorylase activity, following oral supplementation with walnut leaf extract, have been reported [
47]. Due to the important role of hepatic factors in glucose metabolism, it is considered that walnut may be related to reductions in blood glucose levels by inhibiting hepatic gluconeogenesis and stimulating the secretion of pancreatic insulin. It is also suggested that dietary walnut might adjust the metabolic markers in diabetic patients by improvement in endothelial function [
48], whilst Tapsell et al. [
49] assert that PUFAs in walnuts are the effective component for decreasing FBG, plasma insulin, and HbA1c in diabetic patients.
The present study represents the first comprehensive meta-analysis to have examined the effect of JRLE supplementation on circulating lipid and glucose concentrations in T2DM patients. However, there may be multiple limitations in this study. First, the sample size of the included studies was not sufficiently large to confidently detect significant effects. Second, the effects of different forms of JRLE supplements were not adequately examined. Further investigations are warranted to address questions on efficacy, bioavailability, and complete metabolite profiles. Moreover, suitably powered RCTs are needed to establish the clinical efficacy and utility of JRLE. Finally, since all of the participants in the current study were Iranian, the results are not generalizable to other nations.
CONCLUSION
In conclusion, JRLE supplementation seems to favorably affect serum levels of glucose, as well as fasting insulin levels, but it did not influence lipid profile concentrations. This present systematic review and meta-analysis may strengthen the available evidence of JRLE as an alternative adjunctive therapy to better control glycemic targets and lipid parameters.
Student’s Scientific Research Center
NOTES
-
Funding: This study was supported by the Student’s Scientific Research Center, Tehran, Iran.
-
Conflict of Interest: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Daneshvar M, Mirzababaei A, Abaj F, Mirzaei K.
Formal analysis: Daneshvar M, Mirzababaei A.
Investigation: Daneshvar M, Daneshzad E.
Writing - original draft: Daneshvar M, Abaj F, Mirzababaei A, Hosseininasab D.
Writing - review & editing: Daneshzad E, Clark CCT.
SUPPLEMENTARY MATERIAL
Supplementary Table 1
Results of risk of bias assessment for randomized clinical trials included in the current meta-analysis on the effects of J. regia leaf extract supplementation on glycemic and lipid profile parameters*
cnr-11-120-s001.xls
REFERENCES
- 1. Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011;94:311-321.
- 2. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047-1053.
- 3. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet 2017;389:2239-2251.
- 4. Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab 2009;5:150-159.
- 5. Haffner SM. American Diabetes Association. Management of dyslipidemia in adults with diabetes. Diabetes Care 2003;26(Suppl 1):S83-S86.
- 6. Anders HJ, Huber TB, Isermann B, Schiffer M. CKD in diabetes: diabetic kidney disease versus nondiabetic kidney disease. Nat Rev Nephrol 2018;14:361-377.
- 7. Harati Y. Diabetes and the nervous system. Endocrinol Metab Clin North Am 1996;25:325-359.
- 8. Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013;159:543-551.
- 9. Stevens JW, Khunti K, Harvey R, Johnson M, Preston L, Woods HB, Davies M, Goyder E. Preventing the progression to type 2 diabetes mellitus in adults at high risk: a systematic review and network meta-analysis of lifestyle, pharmacological and surgical interventions. Diabetes Res Clin Pract 2015;107:320-331.
- 10. Bahmani M, Mirhoseini M, Shirzad H, Sedighi M, Shahinfard N, Rafieian-Kopaei M. A review on promising natural agents effective on hyperlipidemia. J Evid Based Complementary Altern Med 2015;20:228-238.
- 11. Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 2014;4:177.
- 12. Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care 2003;26:1277-1294.
- 13. Rabiei K, Ebrahimzadeh MA, Saeedi M, Bahar A, Akha O, Kashi Z. Effects of a hydroalcoholic extract of Juglans regia (walnut) leaves on blood glucose and major cardiovascular risk factors in type 2 diabetic patients: a double-blind, placebo-controlled clinical trial. BMC Complement Altern Med 2018;18:206.
- 14. Zhang YJ, Gan RY, Li S, Zhou Y, Li AN, Xu DP, Li HB. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015;20:21138-21156.
- 15. Abbasi Z, Jelodar G, Geramizadeh B.. Prevention of diabetic complications by walnut leaf extract via changing aldose reductase activity: an experiment in diabetic rat tissue. J Diabetes Res 2020;2020:8982676.
- 16. Catanzaro E, Greco G, Potenza L, Calcabrini C, Fimognari C. Natural products to fight cancer: a focus on Juglans regia
. Toxins (Basel) 2018;10:469.
- 17. Javidanpour S, Tabtabaei SRF, Siahpoosh A, Morovati H, Shahriari A. Comparison of the effects of fresh leaf and peel extracts of walnut (Juglans regia L.) on blood glucose and β-cells of streptozotocin-induced diabetic rats. Vet Res Forum 2012;3:251-255.
- 18. Jelodar G, Mohammadi M, Akbari A, Nazifi S. Cyclohexane extract of walnut leaves improves indices of oxidative stress, total homocysteine and lipids profiles in streptozotocin-induced diabetic rats. Physiol Rep 2020;8:e14348.
- 19. Mohammadi J, Delaviz H, Malekzadeh JM, Roozbehi A. The effect of hydro alcoholic extract of Juglans regia leaves in streptozotocin-nicotinamide induced diabetic rats. Pak J Pharm Sci 2012;25:407-411.
- 20. Mollica A, Zengin G, Locatelli M, Stefanucci A, Macedonio G, Bellagamba G, Onaolapo O, Onaolapo A, Azeez F, Ayileka A, Novellino E. An assessment of the nutraceutical potential of Juglans regia L. leaf powder in diabetic rats. Food Chem Toxicol 2017;107:554-564.
- 21. Parham M, Bagherzadeh M, Asghari M, Akbari H, Hosseini Z, Rafiee M, Vafaeimanesh J. Evaluating the effect of a herb on the control of blood glucose and insulin-resistance in patients with advanced type 2 diabetes (a double-blind clinical trial). Caspian J Intern Med 2020;11:12-20.
- 22. Abdoli M, Dabaghian FH, Goushegir A, Shirazi MT, Nakhjavani M, Shojaii A, Rezvani S, Mahlooji K. Anti-hyperglycemic effect of aqueous extract of Juglans regia L. leaf (walnut leaf) on type 2 diabetic patients: a randomized controlled trial. Adv Integr Med 2017;4:98-102.
- 23. Hosseini S, Jamshidi L, Mehrzadi S, Mohammad K, Najmizadeh AR, Alimoradi H, Huseini HF. Effects of Juglans regia L. leaf extract on hyperglycemia and lipid profiles in type two diabetic patients: a randomized double-blind, placebo-controlled clinical trial. J Ethnopharmacol 2014;152:451-456.
- 24. Hosseini S, Huseini HF, Larijani B, Mohammad K, Najmizadeh A, Nourijelyani K, Jamshidi L. The hypoglycemic effect of Juglans regia leaves aqueous extract in diabetic patients: a first human trial. Daru 2014;22:19.
- 25. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. Cochrane Bias Methods Group. Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928.
- 26. Choi M, Park S, Lee M. L-carnitine’s effect on the biomarkers of metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Nutrients 2020;12:2795.
- 27. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics 2018;74:785-794.
- 28. Ashraf S, Arfeen A, Amjad S, Ahmed Z. Effect of walnut (Juglans regia) consumption on hyperlipidemic adults. Cienc Technol Aliment 2021;41:432-438.
- 29. Newsholme P, Keane KN, Carlessi R, Cruzat V. Oxidative stress pathways in pancreatic β-cells and insulin-sensitive cells and tissues: importance to cell metabolism, function, and dysfunction. Am J Physiol Cell Physiol 2019;317:C420-C433.
- 30. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004;114:1752-1761.
- 31. Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006;440:944-948.
- 32. Zhao MH, Jiang ZT, Liu T, Li R. Flavonoids in Juglans regia L. leaves and evaluation of in vitro antioxidant activity via intracellular and chemical methods. Sci World J 2014;2014:303878.
- 33. Oliveira I, Sousa A, Ferreira IC, Bento A, Estevinho L, Pereira JA. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem Toxicol 2008;46:2326-2331.
- 34. Pereira JA, Oliveira I, Sousa A, Ferreira IC, Bento A, Estevinho L. Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food Chem Toxicol 2008;46:2103-2111.
- 35. Amaral JS, Seabra RM, Andrade PB, Valentão P, Pereira JA, Ferreres F. Phenolic profile in the quality control of walnut (Juglans regia L.) leaves. Food Chem 2004;88:373-379.
- 36. Jalili A, Sadeghzade A. Comparative phenolic profile of Persian walnut (Juglans regia L.) leaves cultivars grown in Iran. Afr J Biochem Res 2012;6:33-38.
- 37. Pereira JA, Oliveira I, Sousa A, Valentão P, Andrade PB, Ferreira IC, Ferreres F, Bento A, Seabra R, Estevinho L. Walnut (Juglans regia L.) leaves: phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem Toxicol 2007;45:2287-2295.
- 38. Yoo KM, Lee CH, Lee H, Moon B, Lee CY. Relative antioxidant and cytoprotective activities of common herbs. Food Chem 2008;106:929-936.
- 39. Ali Asgar M. Anti-diabetic potential of phenolic compounds: a review. Int J Food Prop 2012;16:91-103.
- 40. Aryaeian N, Sedehi SK, Arablou T. Polyphenols and their effects on diabetes management: a review. Med J Islam Repub Iran 2017;31:134.
- 41. Sara HS, Zabiholah K, Reza PA. Morphometric study of the effect of walnut (Juglans regia) leaf extract on cerebrum malformation in offsprings of diabetic rats. Biomed Pharmacol J 2015;8:467-475.
- 42. Ortiz-Andrade RR, García-Jiménez S, Castillo-España P, Ramírez-Avila G, Villalobos-Molina R, Estrada-Soto S. α-glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegiana: an anti-hyperglycemic agent. J Ethnopharmacol 2007;109:48-53.
- 43. Obatomi DK, Bikomo EO, Temple VJ. Anti-diabetic properties of the African mistletoe in streptozotocin-induced diabetic rats. J Ethnopharmacol 1994;43:13-17.
- 44. Esmaeili MA, Yazdanparast R. Hypoglycaemic effect of Teucrium polium: studies with rat pancreatic islets. J Ethnopharmacol 2004;95:27-30.
- 45. Lombardo YB, Chicco AG. Effects of dietary polyunsaturated n-3 fatty acids on dyslipidemia and insulin resistance in rodents and humans. A review. J Nutr Biochem 2006;17:1-13.
- 46. Sharma SB, Nasir A, Prabhu KM, Murthy PS. Antihyperglycemic effect of the fruit-pulp of Eugenia jambolana in experimental diabetes mellitus. J Ethnopharmacol 2006;104:367-373.
- 47. Kamyab H, Hejrati S, Khanavi M, Malihi F, Mohammadirad A, Baeeri M, Esmaily H, Abdollahi M. Hepatic mechanisms of the Walnut antidiabetic effect in mice. Cent Eur J Biol 2010;5:304-309.
- 48. Ma Y, Njike VY, Millet J, Dutta S, Doughty K, Treu JA, Katz DL. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: a randomized controlled crossover trial. Diabetes Care 2010;33:227-232.
- 49. Tapsell LC, Batterham MJ, Teuss G, Tan SY, Dalton S, Quick CJ, Gillen LJ, Charlton KE. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur J Clin Nutr 2009;63:1008-1015.
 , Mojtaba Daneshvar1
, Mojtaba Daneshvar1 , Faezeh Abaj1
, Faezeh Abaj1 , Elnaz Daneshzad2
, Elnaz Daneshzad2 , Dorsa Hosseininasab3
, Dorsa Hosseininasab3 , Cain C. T. Clark4
, Cain C. T. Clark4 , Khadijeh Mirzaei1
, Khadijeh Mirzaei1