ABSTRACT
This study sought to investigate the effects of the enhanced recovery after surgery (ERAS) program on postoperative recovery and nutritional status in patients with colorectal cancer undergoing laparoscopic surgery. A total of 37 patients were included: 19 in the experimental group and 18 in the control group. The experimental group was supplemented with carbohydrate drinks before and after surgery, and the control group was maintained with fasting and water intake in the traditional method. Both care management and nutrition education were implemented for both groups. Patients were evaluated for physical condition, clinical indicators, blood tests, pain, length of stay, nutritional status, and nutrient intake. Use of the ERAS program for the experimental group resulted in shorter length of stay (p = 0.006), less pain (p < 0.001), and a lower rate of malnutrition (p = 0.014) compared with controls. In conclusion, carbohydrate drinks provide great advantages by reducing discomfort, such as pain or thirst, during fasting in patients after colon cancer surgery, helping patients to eat comfortably and actively, minimizing insulin resistance, maintaining nitrogen balance, and reducing infection and anastomosis leakage. For use of ERAS as a standardized program, repeated and expanded research is needed, and a Korean-style ERAS should be prepared by using this approach for various diseases.
-
Keywords: Colon cancer; Enhanced postsurgical recovery; Perioperative care; Nutritional status
INTRODUCTION
In 2019, Korea ranked 4th in the incidence of colorectal cancer, and among 184 countries globally, the rate of colorectal cancer was the highest in Korea at 45 people per 100,000 people [
1]. The known primary causes of colorectal cancer are genetic factors, as well as the lifestyle effects and changes, such as aging of the population, westernized eating habits, smoking, drinking, obesity, and a lack of physical activity [
2,
3]. As a treatment method for colorectal cancer, surgical therapy, chemotherapy, and radiation therapy are performed alone or in combination [
4]. A combination of auxiliary chemotherapy and radiation therapy before and after surgery is typically performed [
4,
5]. With this treatment approach, however, colorectal cancer patients experience many health-related quality of life declines, such as psychological shock from the diagnosis of cancer and treatment that limits daily life because of pain and feeling socially alienated due to placement of a permanent stoma [
6]. Preparation for colorectal cancer surgery is removal of gastric residues to reduce the risk of aspiration; usually fasting and intestinal washing are performed for about 8 h, and fasting is continued long-term even after surgery [
7]. During the treatment process, patients often feel discomfort, including dry mouth and thirst, acute pain, nausea and vomiting, discomfort in urination, and abdominal discomfort [
7,
8].
An enhanced recovery after surgery (ERAS) program has been developed to reduce discomfort by optimizing pre- and postoperative care and to reduce repetitive processes, such as fast meal adaptation, repeat surgery, and readmission [
9]. The ERAS program is a multidisciplinary program consisting of several medical staff participating in the treatment and management of patients before, during, and after surgery for ‘evidence-oriented’ individual medical practices to improve post-operative recovery by reducing the body’s stress response [
9]. It is a new concept in surgical patient care and management that ‘multidisciplinary team’ provides ‘multimodal.’ When the ERAS program was discussed, the term ‘fast-track surgery’ was used interchangeably, but the key goal is quality, not speed, of recovery. Implementation of the ERAS program prevents cellular function deterioration, alleviates loss of muscle mass and strength, and alleviates disturbance of metabolic homeostasis by reducing insulin resistance, which increases due to catecholamine secretion and inflammatory response during and after surgery [
8,
9,
10]. In international studies, supplementation of carbohydrate drinks before surgery reduced discomfort in patients after colon cancer surgery, who showed rapid resilience with this approach [
9,
10]. National studies in Korea have also announced the effect of ERAS programs in increasing quality of life and reducing medical expenses [
11]. In Korea, a safe carbohydrate supplement drink has been developed for patients before surgery. This drink has little residue remaining in the stomach 2 hours after consumption and thus has little effect on anesthesia for surgery, leading to its use for management in various surgical populations.
This study aimed to investigate the effects of the ERAS program on postoperative recovery and nutritional status in patients with colorectal cancer undergoing laparoscopic surgery.
MATERIALS AND METHODS
Patients
This study was conducted in patients admitted to a university hospital in Gyeonggi-do, Korea, for colorectal cancer surgery. The inclusion criteria were as follows: age 20 years or older, histologically confirmed colon cancer, and clinical stage (cTanyN0-2), corresponding to Eastern Cooperative Oncology Group performance status 0–2, and American Society of Anesthesiology (ASA) classification ≤ 3. The exclusion criteria were as follows: previous treatment for colorectal cancer or malignant tumors of other tissues within the past 5 years, confirmed metastasis on imaging and histologic analysis (cTanyNanyM1), planned surgery for intestinal fistula, active gastrointestinal bleeding, and previous low anterior resection surgery. A total of 40 participants were recruited, with randomization of 20 patients each to the experimental and control groups. After 3 patients discontinued the study, they were excluded from the final analysis, which assessed the remaining 37 participants.
Study design
At our research institution, the traditional dietary progress of patients after colorectal cancer surgery is the same as that for the control group. In our study, a soft diet was the post-op diet fasting until day 5 for both the experimental and control groups. The ERAS program used in the experimental group ordered carbohydrate drinks from before surgery to postoperative day 4. Care management and nutrition education were conducted in the same way in both groups. The dietary content was investigated for the first meal before and after surgery and at discharge (
Figure 1). The carbohydrate drinks used in the ERAS program are products only sold in Korea. They contain carbohydrates and some electrolytes, and the specific nutritional ingredients are shown in
Table 1.
Figure 1Flow of study progress.
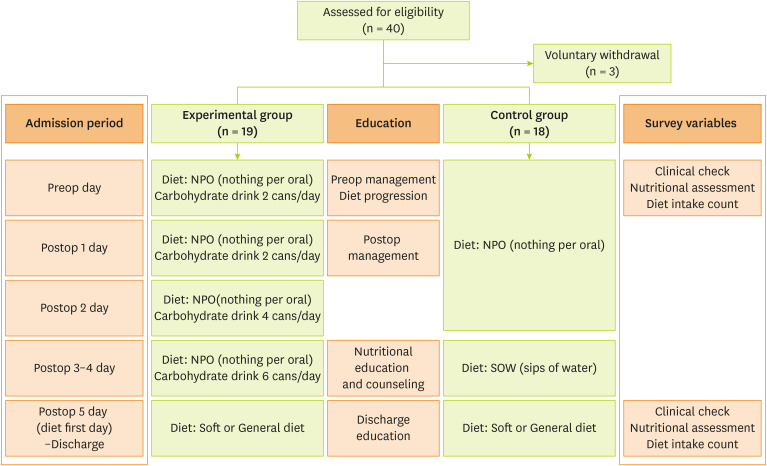
Table 1Information of carbohydrate drink
Table 1
|
Name: No-NPO |
Contents |
|
Volume (mL) |
200 |
|
Energy (kcal) |
100 |
|
Carbohydrate (g) |
25.6 |
|
Sugar (g) |
4 |
|
Fat (g) |
0 |
|
Protein (g) |
0 |
|
Sodium (mg) |
104 |
|
Potassium (mg) |
96 |
Variables
The patient data collected were general characteristics, physical condition (height, weight, and weight loss after surgery), colorectal cancer location, comorbid disease, ASA classification, and length of hospital stay. The ASA categories used in this study were normal health (ASA I), mild systematic disorder (ASA II), and severe systematic disorder (ASA III) [
12].
Changes in hematologic indicators were confirmed by examining patients’ medical records for white blood cell count and concentrations of albumin, lymphocytes, hemoglobin, and hematocrit on the day 7 before surgery and postoperative day 7. Postoperative complications were judged by surgical site inspection. Postoperative pain evaluation was performed using the numeric rating scale, by which patients can select a pain score ranging from 1 to 10 points, and is divided into mild pain (1–3 points), moderate pain (4–6 points), and severe pain (7–10 points).
The energy intake was analyzed based on the usual dietary intake before admission, on postoperative day 5, and on discharge by a clinical dietitian. The nutritional status was evaluated using the Patient-Generated Subjective Global Assessment (PG-SGA) before surgery and at discharge.
Statistical analysis
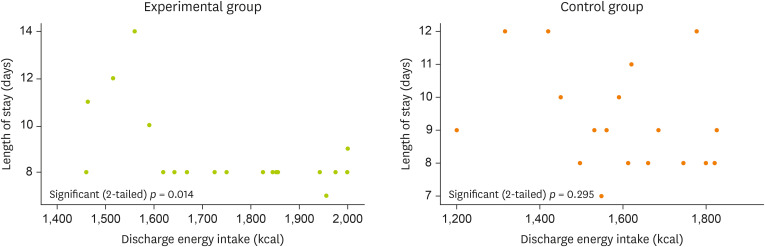
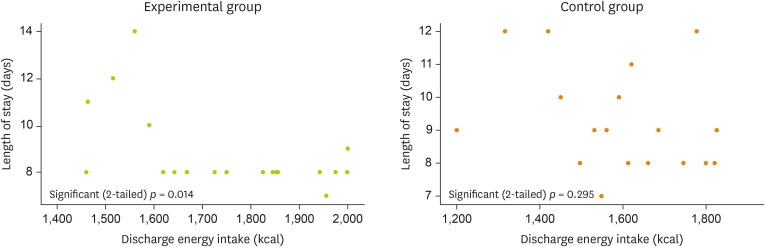
The collected data were analyzed using SPSS, version 25.0 (IBM, Armonk, NY, USA). A homogeneity test was performed by comparing differences between treatment groups using the independent two-sample t-test for continuous variables and the χ2 test for categorical data. The relationship between the length of hospital stays and dietary intake at discharge was analyzed by the Pearson correlation and expressed as a scatterplot. All results confirmed the significance at p < 0.05.
Ethics approval and informed consent
The purpose and content of the surgery were explained, and consent was obtained, and approval was obtained from the Institutional Review Board of Soonchunhyang University Bucheon Hospital (IRB No. 2017-05-012).
RESULTS
Homogeneity test between experimental and control groups
The average age (years) of the study patients was 60.68 ± 8.1 and 62.83 ± 11.09 years in the experimental and control group, respectively. The sex distribution of male and female patients was similar between groups. No differences were noted between groups for patient height and weight. The postoperative weight loss was 1.49 ± 1.35 and 1.65±1.67 kg in the experimental and control group, respectively, which showed more weight loss; however, this difference was not significant. The location of colon cancer was more colon, and comorbidities were mainly diabetes and hypertension. The ASA score of normal health (ASA I) accounted for the largest proportion of ASA scored in both the experimental and control groups with 10 (52.6%) and 8 (44.4%) patients, respectively. No significant between-group differences were found in general characteristics, so the 2 groups were considered to be homogeneous (
Table 2).
Table 2Clinical features in patients
Table 2
|
Characteristics |
Experimental group (n = 19) |
Control group (n = 18) |
p value |
|
Sex |
|
|
0.433 |
|
Male |
9 (47.4) |
10 (55.6) |
|
Female |
10 (52.6) |
8 (44.4) |
|
Age (yr) |
60.68 ± 8.81 |
62.83 ± 11.09 |
0.520 |
|
Height (cm) |
161.58 ± 7.57 |
160.86 ± 8.94 |
0.793 |
|
Preoperative weight (kg) |
65.48 ± 8.54 |
64.51 ± 8.56 |
0.731 |
|
Postoperative weight (kg) |
63.98 ± 8.16 |
62.85 ± 8.35 |
0.679 |
|
Weight loss in hospitalization (kg) |
1.49 ± 1.35 |
1.65 ± 1.67 |
0.750 |
|
Location |
|
|
0.560 |
|
Colon |
10 (52.6) |
10 (55.6) |
|
Sigmoid |
9 (47.4) |
8 (44.4) |
|
Comorbidity |
|
|
0.499 |
|
No |
11 (57.9) |
10 (55.6) |
|
Yes |
9 (47.4) |
8 (44.4) |
|
ASA classification |
|
|
0.532 |
|
I |
10 (52.6) |
8 (44.4) |
|
II |
8 (42.1) |
7 (38.9) |
|
III |
1 (5.3) |
3 (16.7) |
Comparison of perioperative hematologic data
Before the experimental treatment, no statistically significant differences were found between groups on blood test results for white blood cell counts and concentrations of albumin, lymphocyte, hemoglobin, and hematocrit, indicating homogeneity for the 2 groups. After surgery, the white blood cell value slightly increased in the experimental group, with a significant difference in the change in this value between the 2 groups. Surgical site infection, a postoperative complication observation item, was identified in one control group patient. A significant difference was shown between 2 groups for postoperative pain (
p < 0.001), with 3.78 ± 1.32 points in the experimental group, which was lower in pain than 5.67 ± 1.14 points in the control group. The hospitalization period (days) also showed a significant difference in the 2 groups (
p = 0.006), and the experimental group (7.16 ± 2.06) had a shorter hospitalization period than the control group (9.28 ± 1.56) (
Table 3).
Table 3Comparison of perioperative hematologic examination in patients
Table 3
|
Variables |
Experimental group (n = 19) |
Control group (n = 18) |
p value |
|
Albumin change |
1.15 ± 0.62 |
1.17 ± 0.48 |
0.892 |
|
Preop albumin (mg/dL) |
4.25 ± 0.25 |
4.32 ± 0.53 |
0.639 |
|
Postop albumin (mg/dL) |
3.11 ± 0.66 |
3.14 ± 0.66 |
0.857 |
|
White blood cell change (μL) |
−0.23 ± 2.24 |
1.34 ± 1.54 |
0.017 |
|
Preop white blood cell (μL) |
6.10 ± 1.06 |
7.02 ± 1.72 |
0.058 |
|
Postop white blood cell (μL) |
6.33 ± 2.02 |
5.44 ± 1.93 |
0.179 |
|
Lymphocyte change (%) |
10.11 ± 9.57 |
7.37 ± 10.71 |
0.419 |
|
Preop lymphocyte (%) |
32.29 ± 9.12 |
30.39 ± 10.10 |
0.552 |
|
Postop lymphocyte (%) |
22.18 ± 7.30 |
23.02 ± 7.75 |
0.737 |
|
Hemoglobin change (g/dL) |
1.15 ± 0.77 |
1.32 ± 1.08 |
0.585 |
|
Preop hemoglobin (g/dL) |
12.70 ± 1.67 |
13.18 ± 1.84 |
0.414 |
|
Postop hemoglobin (g/dL) |
11.55 ± 1.69 |
11.86 ± 1.24 |
0.530 |
|
Hematocrit change (%) |
3.68 ± 2.13 |
4.01 ± 3.04 |
0.706 |
|
Preop hematocrit (%) |
38.19 ± 3.99 |
39.01 ± 4.45 |
0.560 |
|
Postop hematocrit (%) |
34.51 ± 4.22 |
35.00 ± 3.19 |
0.694 |
|
SSI status |
|
|
0.486 |
|
Yes |
0 (0.0) |
1 (5.6) |
|
No |
19 (100.0) |
17 (94.4) |
|
Postoperative pain status (points) |
3.78 ± 1.32 |
5.67 ± 1.14 |
< 0.001 |
|
Length of hospital stay (days) |
7.16 ± 2.06 |
9.28 ± 1.56 |
< 0.006 |
|
Readmission |
0 (0.0) |
0 (0.0) |
1.000 |
Comparison of perioperative nutritional indicators
Before the experimental treatment, energy intake was 1,815.00 ± 186.66 and 1,830.44 ± 152.92 kcal in the experimental and control group, respectively. Preoperative PG-SGA assessment showed normal nutritional status for most patients, with 17 (94.7%) patients in each group, and thus no significant differences in nutritional characteristics.
The energy intake of the first diet after surgery and the diet on discharge were analyzed. The energy intake of the first meal was 638.26 ± 194.07 and 415.11 ± 183.71 kcal in the experimental and control group, respectively, with higher intakes in the experimental group (
p = 0.001). The energy intake on the discharge day also showed a significant difference between the 2 groups (
p < 0.039), with the experimental group (1,714.26 ± 172.22 kcal) having higher intake than the control group (1,591.89 ± 172.34 kcal). For postoperative nutritional status, the mild-to-moderate malnutrition ratio was higher in the control group at 15.8% versus 27.8% for the experimental group, and the severe malnutrition ratio was 5.3% and 11.1%, respectively, although these differences were not statistically significant (
Table 4).
Table 4Comparison of perioperative diet intake and nutritional status in patients
Table 4
|
Variables |
Experimental group (n = 19) |
Control group (n = 18) |
p value |
|
Preop energy intake (kcal) |
1,815.00 ± 186.66 |
1,830.44 ± 152.92 |
0.785 |
|
Diet first day energy intake (kcal) |
638.26 ± 194.07 |
415.11 ± 183.71 |
0.001 |
|
Discharge energy intake (kcal) |
1,714.26 ± 172.22 |
1,591.89 ± 174.34 |
0.039 |
|
Preop PG-SGA |
|
|
0.969 |
|
Normal |
17 (94.7) |
17 (94.4) |
|
Mild-moderate malnutrition |
1 (5.3) |
1 (5.6) |
|
Severe malnutrition |
0 (0.0) |
0 (0.0) |
|
Discharge PG-SGA |
|
|
0.491 |
|
Normal |
15 (78.9) |
11 (61.1) |
|
Mild-moderate malnutrition |
3 (15.8) |
5 (27.8) |
|
Severe malnutrition |
1 (5.3) |
2 (11.1) |
Relationship between length of hospital stay and diet intake
No between-group differences were observed for the occurrence of postoperative complications, so the correlation between the number of days of hospital stay and the energy intake at discharge was analyzed as an effect variable. In the experimental group, a linear correlation with the length of hospital stay and energy intake was found as a significant result (
p = 0.014), but this correlation was not found for the control group (
p = 0.295) (
Figure 2).
Figure 2The Correlation analysis between length of stay and discharge energy intake in 2 groups.
DISCUSSION
In the overall treatment process, the concept of the ERAS program has emerged as one of the multifaceted approaches, rather than being limited to only surgical techniques. Since it was first proposed in the late 1990s, the ERAS program has now been used for surgical patients in many fields [
13]. In hospitals, cooperation and systematic support between multidisciplinary teams is essential, and the ERAS program can be safely implemented to the extent that it is mainly used based on standardized surgical guidelines (Critical Pathway). However, according to a report by Kim et al. [
14] in Korea, only 29 respondents answered that they used the ERAS program as medical staff working at university hospitals and that the program was mainly used for patients with digestive diseases. This result was very meaningful in that the reasons for not using the ERAS program were the lack of program recognition and the difficulty of implementing this type of detailed program [
14]. In contrast, the ERAS program is actively used in surgeries, such as treatment of biliary pancreatic acid, thyroid disease, pediatric surgery, and vascular surgery. However, the ERAS program is only used in very limited fields in Korea, with no recent research [
15,
16]. Therefore, this study was conducted as a pilot study to develop a standardized ERAS program for colon cancer surgical patients and to improve patient recovery. The effect of the ERAS program was evaluated by supplementing patients with a carbohydrate diet during fasting and using standard education programs.
The experimental and control group patients were compared for the presence of complications and blood test results, which showed no significant differences; most of the blood tests were within the normal range before and after surgery. This finding was the same as the conclusion reached by in the study of Kim et al. [
17], which showed that the frequency of complications and readmission rate did not differ between the experimental and control groups. However, the experimental group experienced a shorter length of stay and significantly less pain. In another previous study [
18], the actual gas-out time was faster, so the meals could be advanced, and as a result, the length of stay was interpreted as a shorter period. Previous studies have decreased the length of stay until the progression to a soft diet by using the ERAS program. In this study, the length of stay in the experimental group was shorter even though the start date of the soft diet was the same in both groups. The reason for this finding was thought to be because postoperative carbohydrate drinks have reduced discomfort such as pain and thirst during fasting and helped patients to eat comfortably and actively. In addition, analysis showed that the average length of stay was 2–4 days earlier than in previous studies because the postoperative management education program was concurrently provided to relieve anxiety and fear of postoperative complications. Based on these findings, use of the ERAS program is desirable as a complex program that includes evaluation indicators and tools, diet progression, education, and counseling for various surgical approaches to treat disease.
The strength of this study is that the nutritional status was compared and evaluated. Most previous studies analyzed length of stay and patient pain or satisfaction to indicate early recovery; in contrast, this study presented results of the analysis of the patient’s actual nutritional intake status. In other words, in the experimental group, the energy intake at discharge was higher, the number of malnourished patients was lower, and a significant relationship between energy intake and length of stay was observed. In general, it is known that the poorer the patient’s nutritional status, the higher the incidence and mortality of complications, delayed wound recovery, and increased medical costs [
19]. Malnutrition is common in gastrointestinal cancer patients because it causes both nutrient malabsorption and metabolic disorders. In previous studies, the malnutrition rate of the patients of this study was low at 21.1% in the experimental group and 38.9% in the control group, compared with 62.6% [
20] and 47.0% [
21] after colon cancer surgery, respectively. During the period when the control group maintained fasting through traditional management methods, the experimental group was supplied with sugar and water by nutritional supplementation with carbohydrate drinks. Postoperative carbohydrate supplementation may be related to great benefits in minimizing insulin resistance, maintaining nitrogen balance, and reducing infection and anastomotic leakage [
22].
Most of the ERAS programs for surgical patients in Korea did not include all of the guidelines, and some cases of self-modification occurred, which may be attributed to different circumstances for each hospital and lack of agreement between medical staff. Limitations of this study include the small number of patients, and that the dietary intervention of carbohydrate drink supplementation was the main rather than the application of the program through a multidisciplinary agreement. Verification of the effectiveness of this approach was insufficient in that strict early recovery standards, such as the use of drainage tube and early walking time, were not applied. For future studies, it is necessary to evaluate various factors such as patient adherence, satisfaction, and quality of life and to further evaluate medical expenses for an ERAS program.
CONCLUSION
Use of an ERAS program in colorectal cancer patients showed that the experimental group had shorter length of stay, reduced pain, and low malnutritional status than the control group. To develop into a more standardized program, expanded iterative studies are needed, and a Korean-style ERAS program should be created by applying this approach to various diseases.
NOTES
-
Conflict of Interest: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Yeom J, Lim HS.
Data curation: Yeom J, Lim HS.
Formal analysis: Yeom J, Lim HS.
Investigation: Yeom J, Lim HS.
Methodology: Yeom J, Lim HS.
Project administration: Yeom J, Lim HS.
Resources: Yeom J, Lim HS.
Supervision: Lim HS.
Writing - original draft: Yeom J, Lim HS.
Writing - review & editing: Lim HS.
REFERENCES
- 1. National Cancer Information Center. Cancer prevalence [Internet]. cited 2022 April 2. Available from https://www.cancer.go.kr/lay1/S1T639C641/contents.do
- 2. Sawicki T, Ruszkowska M, Danielewicz A, Niedźwiedzka E, Arłukowicz T, Przybyłowicz KE. A review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers (Basel) 2021;13:2025.
- 3. Chen X, Jansen L, Guo F, Hoffmeister M, Chang-Claude J, Brenner H. Smoking, genetic predisposition, and colorectal cancer risk. Clin Transl Gastroenterol 2021;12:e00317.
- 4. El-Shami K, Oeffinger KC, Erb NL, Willis A, Bretsch JK, Pratt-Chapman ML, Cannady RS, Wong SL, Rose J, Barbour AL, Stein KD, Sharpe KB, Brooks DD, Cowens-Alvarado RL. American Cancer Society colorectal cancer survivorship care guidelines. CA Cancer J Clin 2015;65:428-455.
- 5. Lee Y, Park I, Cho H, Gwak G, Yang K, Bae BN. Effect of adjuvant chemotherapy on elderly stage II high-risk colorectal cancer patients. Ann Coloproctol 2021;37:298-305.
- 6. Sheikh-Wu SF, Anglade D, Gattamorta K, Xiao C, Downs CA. Positive psychology mediates the relationship between symptom frequency and quality of life among colorectal cancer survivors during acute cancer survivorship. Eur J Oncol Nurs 2022;58:102136.
- 7. Koller SE, Bauer KW, Egleston BL, Smith R, Philp MM, Ross HM, Esnaola NF. Comparative effectiveness and risks of bowel preparation before elective colorectal surgery. Ann Surg 2018;267:734-742.
- 8. Jeong G, Kim K, Kwak Y. Quality of life in colorectal cancer patients according to the severity of symptom clusters classification. Asian Oncol Nurs 2014;14:74-83.
- 9. Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, Rockall TA, Young-Fadok TM, Hill AG, Soop M, de Boer HD, Urman RD, Chang GJ, Fichera A, Kessler H, Grass F, Whang EE, Fawcett WJ, Carli F, Lobo DN, Rollins KE, Balfour A, Baldini G, Riedel B, Ljungqvist O. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations: 2018. World J Surg 2019;43:659-695.
- 10. Makaryus R, Miller TE, Gan TJ. Current concepts of fluid management in enhanced recovery pathways. Br J Anaesth 2018;120:376-383.
- 11. Tian Y, Cao S, Liu X, Li L, He Q, Jiang L, Wang X, Chu X, Wang H, Xia L, Ding Y, Mao W, Hui X, Shi Y, Zhang H, Niu Z, Li Z, Jiang H, Kehlet H, Zhou Y. Randomized controlled trial comparing the short-term outcomes of enhanced recovery after surgery and conventional care in laparoscopic distal gastrectomy (GISSG1901). Ann Surg 2022;275:e15-e21.
- 12. Young J, Badgery-Parker T, Dobbins T, Jorgensen M, Gibbs P, Faragher I, Jones I, Currow D. Comparison of ECOG/WHO performance status and ASA score as a measure of functional status. J Pain Symptom Manage 2015;49:258-264.
- 13. Ljungqvist O, de Boer HD, Balfour A, Fawcett WJ, Lobo DN, Nelson G, Scott MJ, Wainwright TW, Demartines N. Opportunities and challenges for the next phase of enhanced recovery after surgery: a review. JAMA Surg 2021;156:775-784.
- 14. Kim EY, Lee IK. Survey and analysis of the application and implementations of Enhanced Recovery after Surgery (ERAS) Program for surgical patients in the major hospitals in Korea. Surg Metab Nutr 2019;10:32-45.
- 15. Bisch SP, Jago CA, Kalogera E, Ganshorn H, Meyer LA, Ramirez PT, Dowdy SC, Nelson G. Outcomes of enhanced recovery after surgery (ERAS) in gynecologic oncology - A systematic review and meta-analysis. Gynecol Oncol 2021;161:46-55.
- 16. Pang Q, Duan L, Jiang Y, Liu H. Oncologic and long-term outcomes of enhanced recovery after surgery in cancer surgeries—a systematic review. World J Surg Oncol 2021;19:191.
- 17. Kim EJ, Park JS. Development and evaluation of Enhanced Recovery After Surgery Program for patients with colorectal cancer surgery. Korean J Adult Nurs 2019;31:677-690.
- 18. Son GM, Kim KH, Cha SR, Yu H, Paik HJ, Joh YG. Clinical effect of clinical pathway for patients with laparoscopic colon surgery. J Pusan Natl Univ Hosp 2012;31:127-137.
- 19. Mueller C, Compher C, Ellen DM. American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: Nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr 2011;35:16-24.
- 20. Yeom JW, Suh YO. The effect of symptom experience, nutritional status, and self care on quality of life in elderly patients with colorectal cancer. Korean J Rehabil Nurs 2019;22:48-57.
- 21. Gillis C, Richer L, Fenton TR, Gramlich L, Keller H, Culos-Reed SN, Sajobi TT, Awasthi R, Carli F. Colorectal cancer patients with malnutrition suffer poor physical and mental health before surgery. Surgery 2021;170:841-847.
- 22. Zhuang CL, Ye XZ, Zhang XD, Chen BC, Yu Z. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum 2013;56:667-678.