ABSTRACT
Studies indicate an association between hyperuricemia (HUA) and metabolic syndrome risk factors. On the other hand, obesity is a major modifiable and independent risk factor for HUA and gout. However, evidence concerning the effects of bariatric surgery on serum uric acid levels is limited and not completely clarified. This retrospective study was carried out with 41 patients who underwent sleeve gastrectomy (n = 26) and Roux-en-Y gastric bypass (n = 15) from September 2019 to October 2021. Anthropometric, clinical, and biochemical data, including uric acid blood urea nitrogen and creatinine fasting blood sugar (FBS), serum triglyceride (TG), and serum cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), were measured preoperatively and postoperative 3, 6 and 12 months. From baseline to 6 and 12 months, bariatric surgery resulted in a significant decrease in serum uric acid of patients with severe obesity (p < 0.001). The decreases in serum FBS, TG, and cholesterol of patients were significant during 6 and 12 months of follow-up (p < 0.05). However, the HDL increase of patients was not statistically significant in 6 and 12 months (p > 0.05). Besides, although patients’ serum level of LDL decreased significantly during the 6 months of follow-up (p = 0.007), it was not significant after 12 months (p = 0.092). Bariatric surgery significantly reduces serum uric acid levels. Therefore, it may be an effective supplementary therapy for lowering serum uric acid concentrations in morbidly obese patients.
-
Keywords: Bariatric surgery; Obesity; Uric acid; Weight loss
INTRODUCTION
Uric acid is the product of purine metabolism and is produced through the enzymatic degradation of hypoxanthine and xanthine. It is mainly synthesized in the liver and excreted through the kidneys and gut [
1,
2,
3]. However, a high percentage of uric acid (90%) is generally re-absorbed and returned to the bloodstream. The serum uric acid concentration is unstable and is affected by genetic and environmental factors. When these factors are disrupted, hyperuricemia (HUA) may be resulted [
4].
HUA has been identified as serum uric acid concentration above 420 or 340 μmol/L in men and women, respectively [
5]. HUA is a precondition for depositing monosodium urate crystals, which is gout’s primary cause and is related to metabolic syndrome, insulin resistance, renal disease, dyslipidemia, type 2 diabetes, liver failure, and cardiac disease [
6,
7,
8,
9]. The prevalence of HUA has rapidly risen among the general population [
10]. As a result, HUA prevention and control have become a serious public health concern.
Evidence indicates an association between HUA and metabolic syndrome risk factors, such as diabetes, high blood pressure, dyslipidemia, insulin resistance, and obesity [
11]. Obesity is a major modifiable and independent risk factor for HUA and gout [
7,
11]. In previous studies, HUA has been associated with body mass index (BMI), waist circumference, and the waist-height ratio [
12].
Several studies have found that weight loss reduces serum urate levels and causes fewer cases of gouty arthritis [
13,
14]. Based on a recent update of American College of Rheumatology guidelines, weight loss is recommended for obese or overweight patients with gout [
7,
15].
Bariatric surgery reduces caloric intake by altering the anatomy of the gastrointestinal tract, and it is the most efficient way to help people lose weight, especially for people with morbid obesity [
16]. Moreover, a significant weight reduction in morbidly obese patients is associated with a substantial decrease in metabolic risks [
17].
Although previous studies have shown a substantial relationship between HUA and weight changes, evidence concerning the effects of bariatric surgery on serum uric acid levels is limited and not completely clarified. Therefore, the current study was conducted to evaluate the serum uric acid concentration in obese subjects before and after bariatric surgery.
MATERIALS AND METHODS
Study design and participants
In this retrospective study, medical records of patients who underwent sleeve gastrectomy (SG) and Roux-en-Y gastric bypass at the Ghadir Mother and Child Hospital, run under Shiraz University of Medical Sciences, between September 2019 to October 2021 were gathered. Adult patients aged 18–65 years with severe obesity which was defined as BMI > 40 kg/m2 or > 35 kg/m2 with at least one associated obesity-related comorbidity, and who had a follow-up of at least 6 months were included. However, patients with incomplete medical records, under 18 years of age, or lost in follow-up before the 6-month follow-up after the operation were excluded. Moreover, patients with a history of smoking, alcohol use, psychiatric problems, gout, thyroid-related diseases and cancer were excluded. Patients were divided into 2 groups based on follow-up duration. One group was those with 3 and 6 months follow-up and the other group was those patients in the first group who were not lost to follow-up with 3, 6 and 12-month follow-ups. All the surgery procedures were performed by the same surgeon. Informed consent was provided from patients. The researchers adhered to the Declaration of Helsinki, and the study protocol was approved by the Shiraz University of Medical Sciences Ethics Committee (IR.SUMS.REC.1401.476).
Data collection
Patients’ data were extracted from the patients’ medical records. For baseline information, patients were visited, and demographic data, including age and sex, type of surgery, history of past medical diseases, and anthropometric measurements, such as weight, BMI, waist, and hip circumferences, were entered into a checklist by a nutritionist. Besides, preoperative serum laboratory parameters of patients, including uric acid, creatinine, blood urea nitrogen (BUN), fasting blood sugar (FBS), triglyceride (TG), cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL), were recorded. Patients were followed 3, 6, and 12 months following the operation, and the same laboratory tests and anthropometric information were recorded in the postoperative visits by the same nutritionist.
Statistical analysis
The statistical analysis was performed with SPSS software (released 2019, IBM SPSS Statistics for Windows, version 26.0; IBM Corp., Armonk, NY, USA). The information was presented by number and percentage (%) or mean ± standard deviation. Besides, repeated measure analysis was used for data interpretation, and a p value lower than 0.05 was considered statistically significant.
RESULTS
Forty-one patients with a mean age of 43.6 ± 6.8 years were included, of which 32 (78.0%) were female. Besides, 26 (63.4%) patients underwent SG while 15 patients (36.6%) underwent gastric bypass. The comorbidities and baseline anthropometric measures of the patients are demonstrated in
Table 1.
Table 1 Baseline information
Table 1
|
Variables |
Data (n = 41) |
|
Age (yr) |
43.6 ± 6.8 |
|
Female |
32 (78.0) |
|
Surgery |
|
|
Gastric bypass |
15 (36.6) |
|
Sleeve gastrectomy |
26 (63.4) |
|
Weight measures |
|
|
Weight (kg) |
113.1 ± 21.0 |
|
BMI (kg/m2) |
43.1 ± 5.9 |
|
Hip circumference (cm) |
133.0 ± 12.2 |
|
Waist circumference (cm) |
126.1 ± 13.8 |
|
Comorbidities |
|
|
Hypothyroidism |
4 (9.8) |
|
Diabetes mellitus |
5 (12.2) |
|
Hyperlipidemia |
4 (9.8) |
|
Cardiovascular disease |
0 (0.0) |
|
Hypertension |
6 (14.6) |
Table 2 shows the weight outcomes of the patients at 3, 6, and 12 months after the operation. Although no patients were lost to follow-up during the 6-month follow-up concerning weight outcomes, 16 patients were lost in the 12-month follow-up. As shown, all anthropometric measures, including weight, BMI, and hip and waist circumferences of the patients, were decreased significantly during the 6 and 12-month follow-ups (all p < 0.001).
Table 2 Weight outcomes in 6- and 12-month of follow-up
Table 2
|
Variables |
6-mon follow-up (n = 41) |
p value |
12-month follow-up (n = 25) |
p value |
|
Weight (kg) |
Preoperative |
113.1 ± 21.0 |
< 0.001 |
Preoperative |
117.7 ± 21.0 |
< 0.001 |
|
3 mon |
92.3 ± 16.2 |
3 mon |
94.3 ± 15.2 |
|
6 mon |
82.8 ± 14.4 |
6 mon |
85.1 ± 12.4 |
|
|
12 mon |
77.7 ± 12.0 |
|
BMI (kg/m2) |
Preoperative |
43.1 ± 5.9 |
< 0.001 |
Preoperative |
44.7 ± 5.7 |
< 0.001 |
|
3 mon |
35.2 ± 4.8 |
3 mon |
36.0 ± 4.4 |
|
6 mon |
31.1 ± 4.4 |
6 mon |
31.9 ± 3.8 |
|
|
12 mon |
29.1 ± 4.1 |
|
Hip circumference (cm) |
Preoperative |
133.0 ± 12.2 |
< 0.001 |
Preoperative |
136.3 ± 12.3 |
< 0.001 |
|
3 mon |
117.3 ± 10.3 |
3 mon |
120.1 ± 9.9 |
|
6 mon |
110.4 ± 10.3 |
6 mon |
113.3 ± 9.5 |
|
|
12 mon |
107.4 ± 9.7 |
|
Waist circumference (cm) |
Preoperative |
126.1 ± 13.8 |
< 0.001 |
Preoperative |
128.1 ± 12.3 |
< 0.001 |
|
3 months |
110.3 ± 11.3 |
3 mon |
111.2 ± 9.8 |
|
6 mon |
103.8 ± 10.6 |
6 mon |
105.4 ± 9.9 |
|
|
12 mon |
102.2 ± 9.0 |
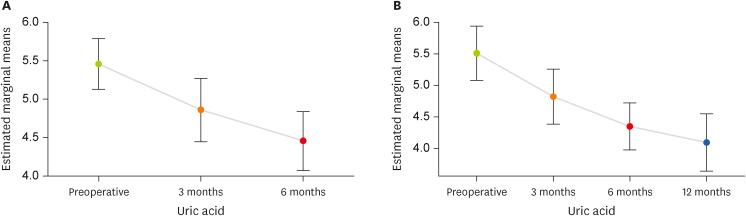
Analysis using repeated measure revealed serum uric acid of patients decreased significantly during the 6-month follow-up (p < 0.001). Regarding the 12-month follow-up, 16 patients were lost to follow-up; however, the decrease remained significant (p < 0.001). As shown in
Figure 1, uric acid of patients had a constantly decreasing trend during the 3 and 12 months of follow-up.
Figure 1 Uric acid changes over 6 months and 12 months of follow-up.
Changes in both BUN and creatinine of patients were not statistically significant during the 6 and 12-month follow-ups (p > 0.05). Of note, the mean serum creatinine of patients was 1.8 mg/dL preoperatively, decreased to a mean of 0.8 mg/dL after 3 months of surgery, and remained 0.8 mg/dL till the 12-month follow-up; although this decrease may be clinically significant, it did not reach a statistical significance.
Moreover, analysis using repeated measure showed that the decreases in serum FBS, TG, and serum cholesterol of patients were significant during 6 and 12 months of follow-up (p < 0.05). However, the HDL increase of patients was not statistically significant (p of 0.417 and 0.378 during 6 and 12-month follow-ups, respectively). Besides, although the patients’ level of LDL decreased significantly during the 6 months of follow-up (p = 0.007), it was not significant after 12 months (p = 0.092) (
Table 3).
Table 3 Changes in serum parameters over 6- and 12-month of follow-up
Table 3
|
Variables |
6-month follow-up |
p value |
12-month follow-up |
p value |
|
Uric acid (mg/dL) |
(n = 41) |
|
< 0.001 |
(n = 25) |
|
< 0.001 |
|
Preoperative |
5.4 ± 1.0 |
Preoperative |
5.5 ± 1.0 |
|
3 mon |
4.8 ± 1.2 |
3 mon |
4.8 ± 1.0 |
|
6 mon |
4.4 ± 1.2 |
6 mon |
4.3 ± 0.9 |
|
|
12 mon |
4.0 ± 1.1 |
|
BUN (mg/dL) |
(n = 41) |
|
0.925 |
(n = 26) |
|
0.561 |
|
Preoperative |
11.8 ± 3.9 |
Preoperative |
10.9 ± 3.9 |
|
3 mon |
11.7 ± 4.1 |
3 mon |
11.9 ± 4.6 |
|
6 mon |
11.6 ± 3.8 |
6 mon |
10.9 ± 3.7 |
|
|
12 mon |
11.8 ± 3.4 |
|
Creatinine (mg/dL) |
(n = 41) |
|
0.532 |
(n = 26) |
|
0.095 |
|
Preoperative |
1.4 ± 2.2 |
Preoperative |
1.8 ± 2.7 |
|
3 mon |
1.0 ± 1.3 |
3 mon |
0.8 ± 0.3 |
|
6 mon |
1.0 ± 1.4 |
6 mon |
0.8 ± 0.2 |
|
|
12 mon |
0.8 ± 0.1 |
|
Fasting blood sugar (mg/dL) |
(n = 41) |
|
0.003 |
(n = 26) |
|
0.004 |
|
Preoperative |
111.2 ± 50.0 |
Preoperative |
107.8 ± 42.9 |
|
3 mon |
94.5 ± 32.3 |
3 mon |
87.9 ± 10.2 |
|
6 mon |
88.3 ± 20.9 |
6 mon |
84.3 ± 19.1 |
|
|
12 mon |
84.3 ± 17.5 |
|
Serum triglyceride (mg/dL) |
(n = 41) |
|
< 0.001 |
(n = 26) |
|
< 0.001 |
|
Preoperative |
157.6 ± 83.1 |
Preoperative |
149.5 ± 46.6 |
|
3 mon |
123.0 ± 48.2 |
3 mon |
121.0 ± 33.2 |
|
6 mon |
106.6 ± 26.8 |
6 mon |
106.5 ± 28.2 |
|
|
12 mon |
105.0 ± 37.2 |
|
Serum cholesterol (mg/dL) |
(n = 39) |
|
0.001 |
(n = 21) |
|
0.007 |
|
Preoperative |
185.8 ± 34.1 |
Preoperative |
188.1 ± 39.0 |
|
3 mon |
171.3 ± 28.7 |
3 mon |
169.2 ± 31.9 |
|
6 mon |
169.7 ± 33.4 |
6 mon |
169.7 ± 37.8 |
|
|
12 mon |
164.5 ± 32.2 |
|
HDL (mg/dL) |
(n = 39) |
|
0.417 |
(n = 21) |
|
0.378 |
|
Preoperative |
45.6 ± 9.3 |
Preoperative |
42.9 ± 8.1 |
|
3 mon |
48.0 ± 12.2 |
3 mon |
44.9 ± 14.1 |
|
6 mon |
46.2 ± 10.1 |
6 mon |
43.5 ± 9.3 |
|
|
12 mon |
47.1 ± 10.5 |
|
LDL (mg/dL) |
(n = 39) |
|
0.007 |
(n = 21) |
|
0.092 |
|
Preoperative |
106.6 ± 29.9 |
Preoperative |
104.3 ± 29.3 |
|
3 mon |
97.3 ± 23.7 |
3 mon |
98.4 ± 25.6 |
|
6 mon |
93.0 ± 28.0 |
6 mon |
92.3 ± 29.4 |
|
|
12 mon |
88.3 ± 30.1 |
DISCUSSION
HUA is caused by an imbalance between the consumption of exogenous purine and endogenous production and expulsion [
17]. Since HUA is an essential indicator of many diseases [
6,
7,
8,
9], preventing and controlling serum uric acid levels has become a critical clinical solution [
18]. Long-term treatment for HUA will be costly and risky. Therefore, reducing the incidence of HUA requires a better understanding of its risk factors and initiating prevention early [
12].
In previous studies, a positive relationship has been found between serum uric acid levels and obesity risk. Several mechanisms may contribute to the interaction between HUA and obesity. The hepatic and peripheral lipogenesis can be accelerated by HUA leading to obesity, and obesity is also a contributing factor to higher levels of serum uric acid [
7,
19]. Further, obesity may impair renal clearance of uric acid, which may explain its positive relationship with serum uric acid levels.
It is believed that treatment of obesity and decreasing BMI may significantly impact serum uric acid and renal urate excretion and play an essential role in regulating serum uric acid levels [
20]. The process of losing weight is not easy for morbidly obese people, and despite their best efforts, many obese individuals are unable to alter their obesity status. As a result, they may enter a cyclical pattern of losing weight and gaining back, and the important thing to note is that metabolic disorders are closely associated with weight fluctuations in obesity conditions [
11].
Bariatric surgery is the best alternative to losing weight in morbidly obese patients [
21]. In this study, we found that serum uric acid levels of patients with severe obesity decreased significantly 6 and 12 months after the surgery. Similarly, multiple studies have shown that bariatric surgery effectively reduced serum uric acid levels in morbidly obese patients [
18,
22,
23,
24]. Sjöström et al. [
25] found that, as compared to usual care, bariatric surgery also reduced uric acid levels in the long-term follow-up, 2 and 10 years after surgery of patients who underwent gastric surgery compared to the control group. Besides, in a meta-analysis study published in 2019, Yeo et al. [
26] concluded that weight loss after bariatric surgery could reduce serum uric acid levels and decrease gout attacks.
For many years, benzbromarone and allopurinol were the main treatments for lowering urate levels. In some obese patients, these therapies did not seem practical at reducing serum uric acid [
10]. Furthermore, patients with HUA or gout may require long-term medication for the rest of their lives, and medication adherence and side effects are significant concerns since gout and HUA are chronic diseases [
18].
Evidence regarding an association between HUA and metabolic syndrome risk factors, such as high blood pressure, dyslipidemia, diabetes, insulin resistance, and obesity, is growing. [
11]. Yuan et al. [
27] conducted a meta-analysis and reported that each 1 mg/dL increase in the serum uric acid level could raise metabolic syndrome risk by 30%. Obesity is the most common cause of metabolic syndrome [
28]. In this study, we found that after surgery, besides reducing weight and BMI, the other parameters related to the metabolic syndrome such as waist circumference, serum TG levels, cholesterol levels, and FBS, significantly improved. Also, Aguilar-Olivos et al. [
29], in a review study, revealed that bariatric surgery has beneficial effects on weight, insulin resistance, alterations in glucose metabolism, and hypertension. Even though bariatric surgery is currently used exclusively for weight loss and not directly for HUA or metabolic syndrome-associated diseases, these conditions are prevalent in most morbidly obese people [
28].
Some limitations of this study should be acknowledged. First, we did not evaluate the dietary intake or physical activity of patients. Therefore, the contribution of any changes in diet contaminant on serum uric acid could not be determined. Secondly, the relatively small sample size of our study is one of its limitations. Lastly, we did not consider the level of alcohol consumption and the number of cigarettes smoked by participants, which both may affect serum uric acid [
20].
In conclusion, this retrospective study demonstrated that bariatric surgery could significantly reduce serum uric acid levels. Thus, bariatric surgery may be an effective supplementary therapy for lowering serum uric acid concentrations in morbidly obese patients and reducing the risk of gout or other HUA-related diseases.
Shiraz University of Medical Scienceshttps://doi.org/10.13039/501100004320
NOTES
-
Funding: The present study was supported by a grant from the Vice-chancellor for Research, Shiraz University of Medical Sciences, Iran.
-
Conflict of Interest: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Haghighat N.
Data curation: Vafa L.
Formal analysis: Kamran H.
Funding acquisition: Amini M.
Investigation: Hosseini SV.
Methodology: Haghighat N.
Project administration: Hosseini SV.
Resources: Amini M.
Software: Kamran H.
Supervision: Amini M.
Validation: Mohammadi Z.
Visualization: Aghakhani L.
Writing - original draft: Aghakhani L.
Writing - review & editing: Haghighat N.
REFERENCES
- 1. Fathallah-Shaykh SA, Cramer MT. Uric acid and the kidney. Pediatr Nephrol 2014;29:999-1008.
- 2. Osgood K, Krakoff J, Thearle M. Serum uric acid predicts both current and future components of the metabolic syndrome. Metab Syndr Relat Disord 2013;11:157-162.
- 3. Sanchez-Lozada LG, Rodriguez-Iturbe B, Kelley EE, Nakagawa T, Madero M, Feig DI, Borghi C, Piani F, Cara-Fuentes G, Bjornstad P, Lanaspa MA, Johnson RJ. Uric acid and hypertension: an update with recommendations. Am J Hypertens 2020;33:583-594.
- 4. Menenakos E, Doulami G, Tzanetakou IP, Natoudi M, Kokoroskos N, Almpanopoulos K, Leandros E, Zografos G, Theodorou D. The use of serum uric acid concentration as an indicator of laparoscopic sleeve gastrectomy success. Int Surg 2015;100:173-179.
- 5. Wang H, Wang L, Xie R, Dai W, Gao C, Shen P, Huang X, Zhang F, Yang X, Ji G. Association of serum uric acid with body mass index: a cross-sectional study from Jiangsu Province, China. Iran J Public Health 2014;43:1503-1509.
- 6. He H, Pan L, Ren X, Wang D, Du J, Cui Z, Zhao J, Wang H, Wang X, Liu F, Pa L, Peng X, Wang Y, Yu C, Shan G. The effect of body weight and alcohol consumption on hyperuricemia and their population attributable fractions: a national health survey in China. Obes Facts 2022;15:216-227.
- 7. Xu C, Wen J, Yang H, You Y, Zhan D, Yu J, Fu L, Zhang T, Liu Y, Yan T. Factors influencing early serum uric acid fluctuation after bariatric surgery in patients with hyperuricemia. Obes Surg 2021;31:4356-4362.
- 8. Hong C, Zhang Q, Chen Y, Lu Y, Chen L, He Y, Li J, Ma S, Jiang J, Zhang X, Hu J, Ding Y, Zhang M, Peng H. Elevated uric acid mediates the effect of obesity on hypertension development: a causal mediation analysis in a prospective longitudinal study. Clin Epidemiol 2022;14:463-473.
- 9. Dehghan A, van Hoek M, Sijbrands EJ, Hofman A, Witteman JC. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care 2008;31:361-362.
- 10. Zhou J, Wang Y, Lian F, Chen D, Qiu Q, Xu H, Liang L, Yang X. Physical exercises and weight loss in obese patients help to improve uric acid. Oncotarget 2017;8:94893-94899.
- 11. Gao B, Zhou J, Ge J, Zhang Y, Chen F, Lau WB, Wan Y, Zhang N, Xing Y, Wang L, Fu J, Li X, Jia H, Zhao X, Ji Q. Association of maximum weight with hyperuricemia risk: a retrospective study of 21,414 Chinese people. PLoS One 2012;7:e51186.
- 12. Zhou Z, Li K, Li X, Luan R, Zhou R. Independent and joint associations of body mass index, waist circumference, waist-height ratio and their changes with risks of hyperuricemia in middle-aged and older Chinese individuals: a population-based nationwide cohort study. Nutr Metab (Lond) 2021;18:62.
- 13. Maglio C, Peltonen M, Neovius M, Jacobson P, Jacobsson L, Rudin A, Carlsson LM. Effects of bariatric surgery on gout incidence in the Swedish Obese Subjects study: a non-randomised, prospective, controlled intervention trial. Ann Rheum Dis 2017;76:688-693.
- 14. Dessein PH, Shipton EA, Stanwix AE, Joffe BI, Ramokgadi J. Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis 2000;59:539-543.
- 15. FitzGerald JD, Dalbeth N, Mikuls T, Brignardello-Petersen R, Guyatt G, Abeles AM, Gelber AC, Harrold LR, Khanna D, King C, Levy G, Libbey C, Mount D, Pillinger MH, Rosenthal A, Singh JA, Sims JE, Smith BJ, Wenger NS, Bae SS, Danve A, Khanna PP, Kim SC, Lenert A, Poon S, Qasim A, Sehra ST, Sharma TS, Toprover M, Turgunbaev M, Zeng L, Zhang MA, Turner AS, Neogi T. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken) 2020;72:744-760.
- 16. DeMaria EJ. Bariatric surgery for morbid obesity. N Engl J Med 2007;356:2176-2183.
- 17. Tana C, Busetto L, Di Vincenzo A, Ricci F, Ticinesi A, Lauretani F, Nouvenne A, Giamberardino MA, Cipollone F, Vettor R, Meschi T. Management of hyperuricemia and gout in obese patients undergoing bariatric surgery. Postgrad Med 2018;130:523-535.
- 18. Liu W, Zhang H, Han X, Zhang P, Mao Z. Uric acid level changes after bariatric surgery in obese subjects with type 2 diabetes mellitus. Ann Transl Med 2019;7:332.
- 19. İnanir M. Serum uric acid (SUA) in morbidly obese patients and its relationship with metabolic syndrome. Aging Male 2020;23:1165-1169.
- 20. Ishizaka N, Ishizaka Y, Toda A, Tani M, Koike K, Yamakado M, Nagai R. Changes in waist circumference and body mass index in relation to changes in serum uric acid in Japanese individuals. J Rheumatol 2010;37:410-416.
- 21. Abdul Wahab R, le Roux CW. A review on the beneficial effects of bariatric surgery in the management of obesity. Expert Rev Endocrinol Metab 2022;17:435-446.
- 22. Qu X, Zheng L, Zu B, Jia B, Lin W. Prevalence and clinical predictors of hyperuricemia in chinese bariatric surgery patients. Obes Surg 2022;32:1508-1515.
- 23. Zetu C, Popa S, Golli AL, Condurache A, Munteanu R. Long-term improvement of dyslipidaemia, hyperuricemia and metabolic syndrome in patients undergoing laparoscopic sleeve gastrectomy. Arch Endocrinol Metab 2021;64:704-709.
- 24. Lu J, Bai Z, Chen Y, Li Y, Tang M, Wang N, Zhu X, Dai H, Zhang W. Effects of bariatric surgery on serum uric acid in people with obesity with or without hyperuricaemia and gout: a retrospective analysis. Rheumatology (Oxford) 2021;60:3628-3634.
- 25. Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, Sullivan M, Wedel H. Swedish Obese Subjects Study Scientific Group. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683-2693.
- 26. Yeo C, Kaushal S, Lim B, Syn N, Oo AM, Rao J, Koura A, Yeo D. Impact of bariatric surgery on serum uric acid levels and the incidence of gout—a meta-analysis. Obes Rev 2019;20:1759-1770.
- 27. Yuan H, Yu C, Li X, Sun L, Zhu X, Zhao C, Zhang Z, Yang Z. Serum uric acid levels and risk of metabolic syndrome: a dose-response meta-analysis of prospective studies. J Clin Endocrinol Metab 2015;100:4198-4207.
- 28. Cordero P, Li J, Oben JA. Bariatric surgery as a treatment for metabolic syndrome. J R Coll Physicians Edinb 2017;47:364-368.
- 29. Aguilar-Olivos NE, Almeda-Valdes P, Aguilar-Salinas CA, Uribe M, Méndez-Sánchez N. The role of bariatric surgery in the management of nonalcoholic fatty liver disease and metabolic syndrome. Metabolism 2016;65:1196-1207.
 , Masoud Amini1
, Masoud Amini1 , Hooman Kamran1
, Hooman Kamran1 , Ladan Aghakhani1
, Ladan Aghakhani1 , Seyed Vahid Hosseini2
, Seyed Vahid Hosseini2 , Zahra Mohammadi1
, Zahra Mohammadi1 , Neda Haghighat1
, Neda Haghighat1