ABSTRACT
Probiotics affect biomarkers indicative of bone formation, such as alkaline phosphatase (ALP), calcium status, bone mineralization, bone turnover markers and metabolism. This study aims to investigate the effects of synbiotic on gastrointestinal (GI) disorder, bone complications and anemia in hemodialysis (HD) patients. In this randomized, double-blind, placebo-controlled clinical trial study, HD patients received 2 symbiotic (n = 19) or placebo (n = 17) capsules daily for 12 weeks. GI function, serum levels of bone-specific biomarkers, and serum levels of anemia-specific biomarkers were assessed at the beginning and the end of study. GI function was assessed with gastrointestinal symptom rating scale questionnaire. The data were analyzed using SPSS. At the end of this study, parathyroid hormone levels decreased significantly in the synbiotic group (p = 0.039); however, in comparison to placebo group, the difference was not significant. Decrease of ALP levels in the synbiotic group were not statistically significant. However, a significant difference was seen between the 2 groups at the end of intervention (p = 0.037). Improvement in GI symptoms was observed in both groups, but the reduction rate was higher in the synbiotic group. Additionally, at the end of the study, a significant difference between the 2 groups was observed (p < 0.05). No statistically significant difference was observed in the levels of other factors within each group and between the 2 groups (p > 0.05). Symbiotic supplements after 12 weeks led to an improvement in GI function and ALP levels in HD patients. Further investigation into bone-mineral disorders in HD patients is necessary.
-
Trial Registration
-
Keywords: Probiotics; Chronic kidney failure; Parathyroid hormone; Alkaline phosphatase
INTRODUCTION
Previous studies estimate the global prevalence of chronic kidney disease (CKD) to be between 8% and 16% [
1]. The end-stage of CKD typically necessitates alternative treatment, often involving hemodialysis (HD) [
2]. Among the first disorders of mineral metabolism in patients with CKD is an increase in the blood level of parathyroid hormone (PTH). Changes in serum calcium and phosphorus levels in CKD and end-stage renal disease (ESRD) lead to disturbances in PTH secretion, resulting in secondary hyperparathyroidism and complications, such as bone pain and fractures [
3]. One of the methods for evaluating PTH status in HD patients is measuring the serum level of alkaline phosphatase (ALP), which serves as a marker for assessing uremic osteodystrophy [
4]. An increase in PTH secretion along with an increase in bone ALP isoenzyme, has been observed in these patients [
5].
In patients with ESRD, the frequency of certain bacterial families such as
Lactobacilli and
Protella decreases. These bacterial families produce enzymes that help form short-chain fatty acids [
6]. Several studies have demonstrated a beneficial impact of probiotics on bone metabolism. Key mechanisms involve enhancing mineral solubility through the production of short-chain fatty acids, synthesizing phytase enzymes to counteract the mineral-depressing effect of phytate, decreasing intestinal inflammation to promote increased bone mass, and breaking down glycoside bonds in food within the intestines by
Lactobacillus and
Bifidobacteria. These actions ultimately result in improved mineral bioavailability and density [
7]. Probiotics have been shown to influence calcium absorption in the intestine, thereby affecting overall calcium status and bone mineralization [
8]. Moreover, probiotics have been found to impact biomarkers indicative of bone formation, such as bone-specific ALP and N-terminal propeptide of type I procollagen [
9]. According to the results of the Harahap study, consumption of probiotic
Lactobacillus acidophilus appeared to reduce changes in bone turnover markers [
10]. In the study by Vanitchanont et al. [
11], after 12 weeks, the multispecies probiotic group exhibited a significant decrease in the serum bone resorption marker (C-terminal telopeptide of type I collagen) in osteopenic postmenopausal women compared to baseline. This suggests that multispecies probiotics may have a preventive effect on bone through their antiresorptive effects [
11].
In previous studies, the effects of high doses of Lactobacillus strains on the improvement of bone complications in patients with HD have not been investigated. The aim of this study was to explore the impact of synbiotic supplementation containing Lactobacillus strains and fructooligosaccharides on GI function, anemia, as well as PTH, ALP, and other serum levels of bone-specific biomarkers through various mechanisms.
MATERIALS AND METHODS
Subjects and study design
This randomized, parallel, double-blind, and controlled clinical trial took place from December 2022 to February 2023 involving HD patients and was conducted at Farabi Hospital in Isfahan. The current study was approved by the Ethics Committee of Kermanshah University of Medical Sciences (IR.KUMS.REC.1401.033) and registered in the Iranian Registry of Clinical Trials (
IRCT20131013014994N7).
Patients regardless of age and gender, who met the following criteria were enrolled in the study: undergoing HD treatment at least twice a week for up to 4 hours per session for a minimum of 6 months; not pregnant or lactating; devoid of immune system deficiency, active cancer, severe GI diseases, human immunodeficiency virus (HIV), or severe chronic illnesses; not using probiotic, prebiotic supplements, or antibiotics for the preceding 4 weeks; free from addiction or alcohol consumption; capable of consuming at least 200 milliliters of water daily. Patients with severe edema, peritoneal dialysis, candidiasis, kidney or other organ transplants during the study; those experiencing side effects from the supplement; or individuals unwilling to continue participating were excluded from the trial.
The sample size was estimated using the standard formula which was used in previous clinical trials [
12]. A minimum sample size of 15 individuals in each group was calculated, which was increased to 21 individuals in each group considering dropout rates. Following the acquisition of informed consent, participants filled out questionnaires regarding demographics, medication usage, medical history, and smoking status. Participants were allocated to the synbiotic and control groups (each with 21 participants) through 4-person blocked randomization based on age and gender using randomized allocation software (RAS). The randomization process, enrollment of participants, and assignment of participants to interventions were performed by a statistical consultant. Placebo and synbiotic capsules were coded as A and B by the supplement provider company and until the completion of the data analysis, they were kept in a sealed envelope with the company. Both patients and researchers were blind to this process until the end of the data analysis.
Participants in synbiotic group received 2 synbiotic capsules (GeriLact; Zist Takhmir Co., Tehran, Iran; each capsule containing 109 colony-forming unit (CFU) probiotics including Lactobacillus rhamnosus, Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus fermentum, Lactobacillus plantarum, Lactobacillus gasseri, and 21 mg of prebiotic fructooligosaccharides) and participants in control group received 2 placebo capsules (each capsule containing 350 mg of inulin and maltodextrin) every day after lunch and dinner for 12 weeks. Patient adherence to the consumption was followed up weekly by telephone contacts. Before commencing the study, all patients were asked to report any consumption of food items containing probiotic strains or probiotic supplements during the study period. This information would be regarded as a potential confounding factor in the analysis at the conclusion of the study.
Data collection
At the beginning and the end of the study, information regarding dietary intake, physical activity, and anthropometric measurements of the patients were evaluated by the researcher. After necessary instructions, dietary intake was assessed by patients using a food record questionnaire for 3 days (2 dialysis days and 1 regular day) and the accuracy of the recorded data was checked by the researcher. Dietary intakes were assessed using the Nutritionist IV software (version 4.1, 1997; First DataBank, San Bruno, CA, USA) [
13]. The outcomes of this study included assessing gastrointestinal function, measuring serum levels of PTH, ALP, calcium, phosphorus, platelets, hematocrit, ferritin, hemoglobin, Total iron binding capacity (TIBC), albumin (at the beginning and the end of the study using participants’ blood samples taken after a 12-hour fasting before dialysis session), exploring the relationship between serum levels of PTH and calcium and phosphorus levels, and the association between advanced glycation end products (AGEs) and glycated hemoglobin (HbA1c) with gastrointestinal disorders in patients.
To assess gastrointestinal function at the beginning and the end of the study, the persian version of the gastrointestinal symptom rating scale (GSRS) was utilized, which demonstrated acceptable validity and reliability [
14]. This questionnaire consisted of 15 questions evaluating 5 categories of gastrointestinal disturbances including indigestion, abdominal pain, reflux, diarrhea, and constipation on a Likert scale from no (score: 1) to very severe (score: 7) discomfort. This questionnaire was initially translated into Persian by researchers and evaluated by a gastroenterologist. The total score was calculated from the average scores, where an increase indicated symptom severity. Physical activity levels at the beginning and the end of the study were assessed using the international physical activity questionnaire (IPAQ), which demonstrated acceptable validity and reliability [
15]. This questionnaire included 7 questions and was considered if the patient had continuous physical activity with vigorous, moderate degree and walking for at least 10 minutes during the previous 7 days. A coefficient of 8 was assigned for vigorous activity, 4 for moderate activity, and 3 for walking. After determining these coefficients, the duration allocated to each type of activity per day was multiplied by the number of days assigned to that activity per week. The sum of these values was considered the patients’ physical activity score. Plasma samples were stored at −80°C for AGE level determination. Plasma AGEs were quantified using a human ELISA kit (ZellBio GmbH, Lonsee, Germany) [
13].
Statistical analysis was carried out using the SPSS software (version 26; IBM Corp., Armonk, NY, USA). The Shapiro-Wilk test was employed to assess the distribution of the data. The data were analyzed through an independent sample t-test, χ2 test, and paired t-test. At the end of the study, the analysis of covariance test was employed to adjust the effect of confounding variables and baseline values. The Pearson’s correlation test was used to examine the relationships between variables. A significance level of less than 0.05 was considered for all analyses. If the study groups differed significantly in terms of confounding factors (dietary intake, energy intake, protein, fat and carbohydrate intake, physical activity, medications, duration of HD treatment, number of HD sessions per week, medical history, and use of probiotic-containing products during the study period), adjustments were made in the statistical analyses.
RESULTS
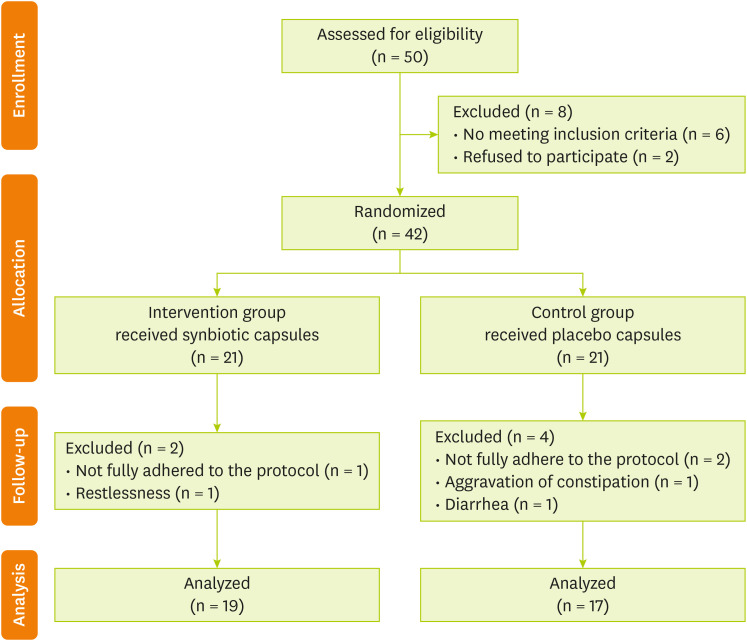
A total of 50 HD patients were evaluated. After screening and eligibility assessment, ultimately 42 HD patients entered the study (
Figure 1). Participants were randomly assigned into 2 groups: synbiotic and control groups. Each group consisted of 21 individuals. In the synbiotic group, 2 patients were excluded from the study because of non-adherence to the protocol (n = 1) and restlessness following the intake supplement (n = 1). Additionally, in the control group, 4 patients were excluded from the study due to poor adherence to the protocol (n = 2), constipation (n = 1), and diarrhea (n = 1) after taking the capsules. Finally, 19 patients in the synbiotic group and 17 patients in the control group underwent final evaluation after 12 weeks.
Figure 1 Flow diagram of the study.
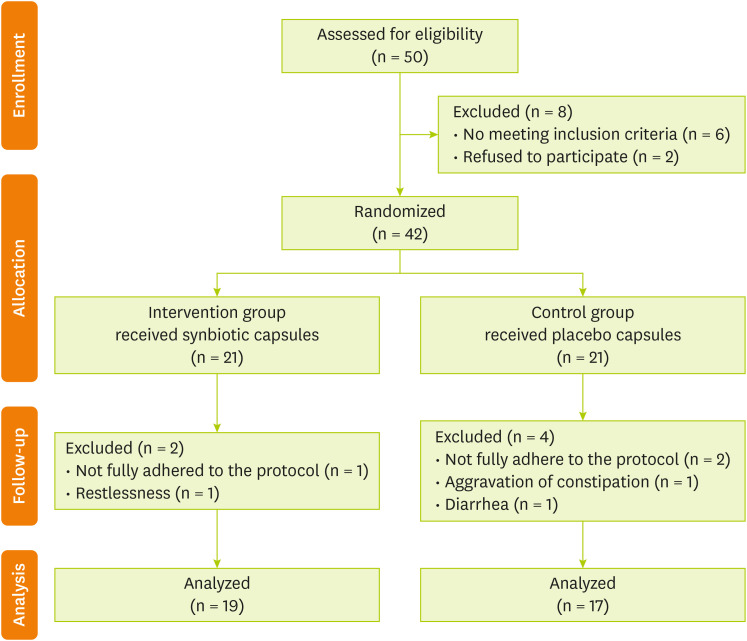
Table 1 demonstrates the general characteristics of HD patients at the beginning of study. Out of all participants, 25 were male (69.4%) and the remaining were female. The mean of age and body mass index of participants were 52.84 ± 17.28 years and 23.42 ± 3.99 kg/m
2 in symbiotic group and 56.12 ± 15.69 years and 22.31 ± 3.84 kg/m
2 in the control group. Regarding the variables shown in the
Table 1, there was not a statistically significant difference between the synbiotic and control groups (p > 0.05). Intake of energy, macronutrients, and micronutrients did not differ significantly between the 2 groups at the beginning and at the end of the study (p > 0.05), except for the intake of saturated fatty acids in the synbiotic group, which showed a significant increase after the intervention compared to the beginning of the study (19.65 ± 5.11 g/day vs. 21.48 ± 5.53 g/day; p = 0.03).
Table 1 Demographic characteristics of participants at baseline of the study
Table 1
|
Group |
Participants (n = 36) |
Synbiotic group (n = 19) |
Control group (n = 17) |
p value |
|
Sex |
|
|
|
0.070 |
|
Male |
25 (69.4) |
14 (73.7) |
11 (64.7) |
|
Female |
11 (30.6) |
5 (26.3) |
6 (35.3) |
|
Age (yr) |
54.39 ± 16.39 |
52.84 ± 17.28 |
56.12 ± 15.69 |
0.557 |
|
Height (cm) |
165.50 ± 11.62 |
165 ± 1.4 |
167 ± 7 |
0.704 |
|
Weight (kg) |
63.49 ± 16.10 |
64.70 ± 17.86 |
62.15 ± 14.33 |
0.642 |
|
Body mass index (kg/m2) |
22.9 ± 3.9 |
23.42 ± 3.99 |
22.31 ± 3.84 |
0.402 |
|
Physical activity (MET-min/Week) |
431.83 ± 753.17 |
439.53 ± 769.73 |
432.24 ± 757.79 |
0.949 |
|
Diabetes |
|
|
|
0.063 |
|
Yes |
12 (33.3) |
6 (31.6) |
6 (35.3) |
|
No |
24 (66.7) |
13 (68.4) |
11 (64.7) |
|
Cardiovascular disease |
|
|
|
0.739 |
|
Yes |
19 (52.8) |
9 (47.4) |
10 (58.8) |
|
No |
17 (47.2) |
10 (52.6) |
7 (41.2) |
|
Smoking |
|
|
|
0.800 |
|
Yes |
10 (27.8) |
8 (42.1) |
2 (11.8) |
|
No |
26 (72.2) |
11 (57.9) |
15 (88.2) |
|
Duration of hemodialysis session (hr) |
3.86 ± 0.351 |
3.89 ± 0.32 |
3.82 ± 0.40 |
0.551 |
|
Duration of hemodialysis treatment (mon) |
57.94 ± 36.86 |
56.42 ± 41.90 |
59.65 ± 31.52 |
0.798 |
Table 2 presents the serum PTH, ALP, and other factors, along with gastrointestinal function, in patients with ESRD before and after the intervention. As shown the serum levels of PTH in the synbiotic group, had a statistically significant decrease at the end of the study (p = 0.039), but after adjusting for confounders, no significant difference was observed between the 2 groups. The synbiotic supplement led to a decrease in serum ALP levels, but it was not statistically significant. However, after adjusting for confounders, a significant difference was observed between the 2 groups at the end of the study (p < 0.05). No statistically significant differences were observed between the 2 groups in terms of serum calcium, phosphorus, albumin, hemoglobin, ferritin, TIBC, hematocrit, and platelets at the end of the study.
Table 2 Blood parameters in hemodialysis patients at baseline and endpoint of the study
Table 2
|
Variable |
Group |
|
|
Synbiotic group (n = 19) |
Control group (n = 17) |
|
Before |
After |
p*
|
Before |
After |
p*
|
p†
|
p‡
|
|
iPTH (pg/mL) |
630.13 ± 127.95 |
479.49 ± 103.68 |
0.039 |
746.62 ± 117.34 |
689.39 ± 126.96 |
0.486 |
0.517 |
0.227 |
|
ALP (U/L) |
495 ± 361.91 |
460.16 ± 355.29 |
0.189 |
539.76 ± 411.04 |
653.18 ± 607.8 |
0.101 |
0.730 |
0.037 |
|
Calcium (mg/dL) |
8.45 ± 0.73 |
8.69 ± 0.63 |
0.230 |
8.63 ± 0.365 |
8.65 ± 0.418 |
0.851 |
0.414 |
0.476 |
|
Phosphorus (mg/dL) |
5.15 ± 1.07 |
5.16 ± 1.03 |
0.982 |
5.6 ± 0.95 |
5.57 ± 1.33 |
0.921 |
0.234 |
0.716 |
|
Albumin (mg/L) |
3.95 ± 0.081 |
3.85 ± 0.076 |
0.149 |
3.80 ± 0.054 |
3.9 ± 0.087 |
0.386 |
0.122 |
0.299 |
|
Hemoglobin (g/dL) |
11.10 ± 1.54 |
10.98 ± 1.68 |
0.669 |
11.05 ± 1.46 |
11.14 ± 1.24 |
0.797 |
0.922 |
0.832 |
|
Ferritin (mg/L) |
344.46 ± 111.36 |
267.60 ± 78.89 |
0.401 |
326.66 ± 99.76 |
278.15 ± 68.99 |
0.563 |
0.939 |
0.822 |
|
TIBC (mg/dL) |
307.84 ± 139.57 |
289.89 ± 136.84 |
0.201 |
316.47 ± 123.57 |
305.29 ± 116.46 |
0.250 |
0.846 |
0.642 |
|
HCT (%) |
31.9 ± 8.82 |
33.6 ± 9.43 |
0.055 |
34.24 ± 4.44 |
36.02 ± 3.62 |
0.145 |
0.330 |
0.712 |
|
Platelet (mm3) |
157,473.68 ± 61,318.26 |
166,421.05 ± 57,811.29 |
0.420 |
176,411.76 ± 57,597.8 |
163,647.06 ± 30,638.9 |
0.217 |
0.348 |
0.283 |
|
AGEs (ng/L) |
711.96 ± 248.49 |
870.51 ± 300.78 |
< 0.001 |
741.36 ± 272.80 |
847.98 ± 245.36 |
0.001 |
0.737 |
0.272 |
|
HbA1c (%) |
6.44 ± 1.57 |
6.54 ± 1.77 |
0.517 |
7.20 ± 2.20 |
7.08 ± 1.91 |
0.700 |
0.235 |
0.991 |
Table 3 showed the improvement in all gastrointestinal symptoms in both the synbiotic and placebo groups after the intervention. In general, the severity of gastrointestinal symptoms improved in both the synbiotic and control groups. However, this reduction was more pronounced in the synbiotic group (p < 0.05). After adjusting for confounders at the end of the study, all symptoms, except for abdominal pain, had a statistically significant difference between the 2 groups (p < 0.05). As shown in
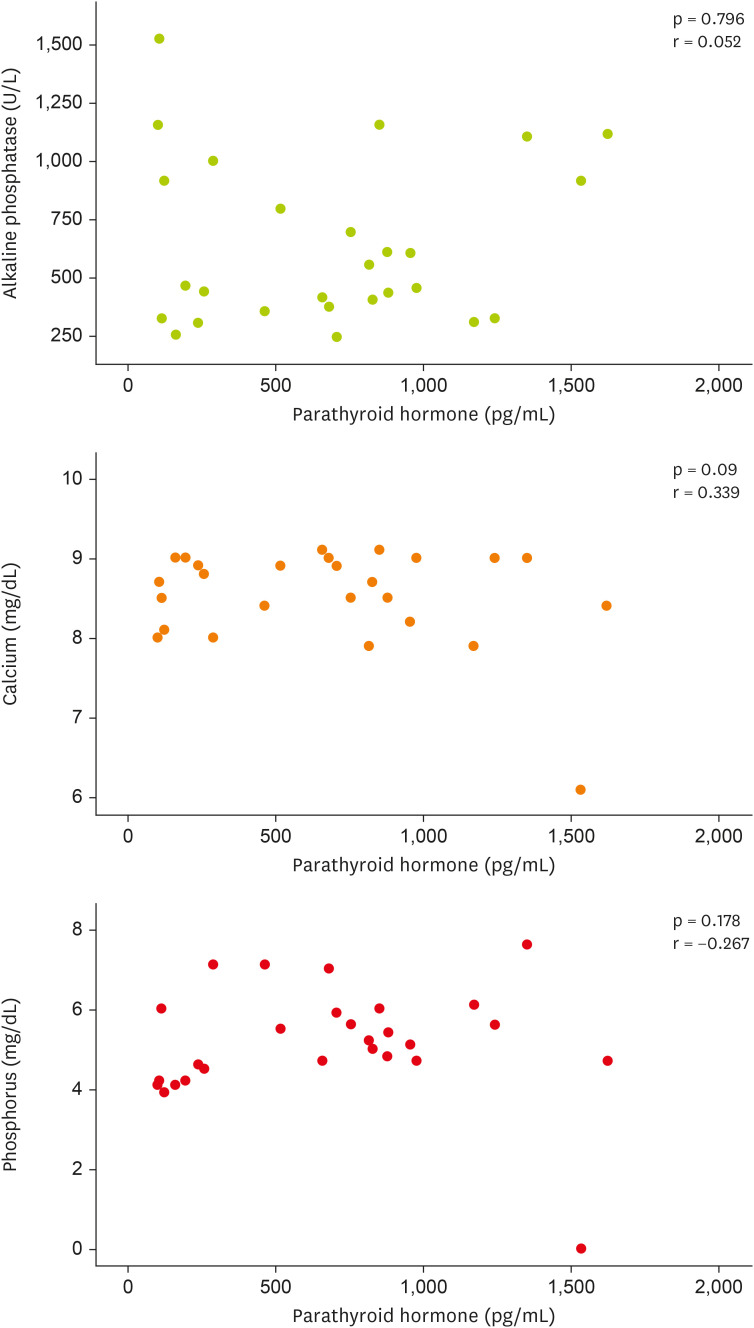
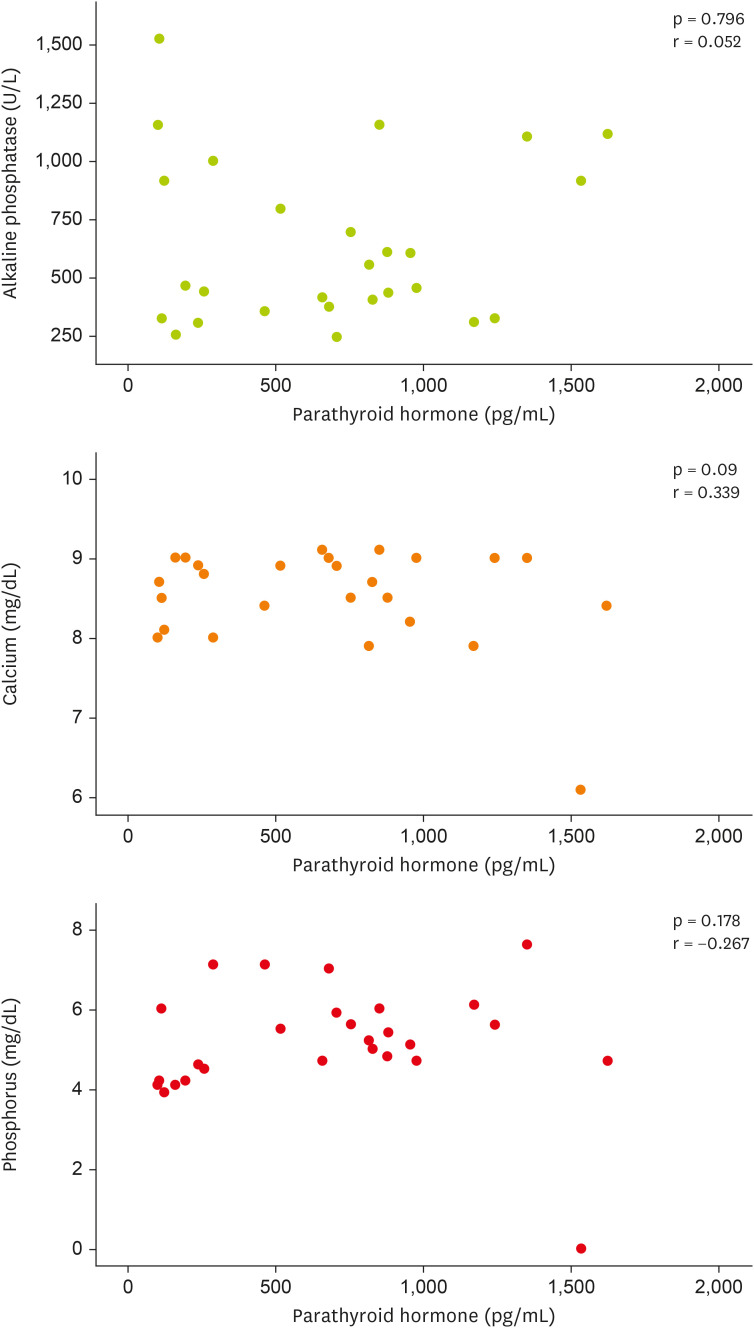
Figure 2 (scatter plot), no statistically significant correlation was observed between the levels of PTH and ALP, calcium, and phosphorus.
Table 3 Gastrointestinal symptoms of patients at baseline and endpoint of the study
Table 3
|
Variable |
Group |
|
|
Synbiotic group (n = 19) |
Control group (n = 17) |
|
Before |
After |
p*
|
Before |
After |
p*
|
p†
|
p‡
|
|
GSRS |
2.94 ± 1.00 |
1.68 ± 0.49 |
< 0.001 |
2.76 ± 0.71 |
2.34 ± 0.80 |
0.001 |
0.549 |
< 0.001 |
|
Abdominal pain |
8.74 ± 5.17 |
5.47 ± 1.45 |
0.003 |
5.35 ± 2.15 |
4.29 ± 1.45 |
0.011 |
0.016 |
0.878 |
|
Reflux |
3.95 ± 3.03 |
2.79 ± 1.72 |
0.013 |
4.29 ± 3.28 |
3.88 ± 3.28 |
0.049 |
0.743 |
0.041 |
|
Diarrhea |
4.42 ± 2.21 |
3.31 ± 1.10 |
0.0038 |
4.12 ± 2.15 |
4.35 ± 1.57 |
0.072 |
0.585 |
0.019 |
|
Constipation |
11.57 ± 6.34 |
5.89 ± 3.69 |
< 0.001 |
10.76 ± 4.77 |
9.23 ± 4.75 |
0.007 |
0.669 |
0.001 |
|
Indigestion |
15.31 ± 8.23 |
7.68 ± 3.30 |
< 0.001 |
16.11 ± 6.36 |
13.23 ± 7.04 |
0.009 |
0.748 |
0.001 |
Figure 2 Correlation between parathyroid hormone, serum levels of alkaline phosphatase, calcium, and phosphorus at baseline and endpoint of the study in hemodialysis patients.
The results of the investigation into the relationship between AGEs and HbA1c with gastrointestinal disorders in ESRD patients undergoing HD treatment are reported in
Table 4. After data analysis, a significant correlation was found between serum HbA1c levels and constipation at the beginning and at the end of the study (p < 0.05), but no other correlations were observed (p > 0.05).
Table 4 Correlation between AGEs, HbA1c, and gastrointestinal disorders at baseline and endpoint of study
Table 4
|
Variable |
Group |
|
GSRS |
Abdominal pain |
Reflux |
Diarrhea |
Constipation |
Indigestion |
|
r |
p |
r |
p |
r |
p |
r |
p |
r |
p |
r |
p |
|
AGEs (ng/L) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Before |
0.14 |
0.415 |
0.034 |
0.842 |
0.105 |
0.538 |
−0.056 |
0.743 |
0.304 |
0.071 |
−0.03 |
0.859 |
|
After |
0.06 |
0.725 |
0.009 |
0.957 |
0.037 |
0.830 |
−0.074 |
0.666 |
0.140 |
0.415 |
0.001 |
0.994 |
|
HbA1c (%) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Before |
0.052 |
0.761 |
−0.143 |
0.405 |
−0.261 |
0.122 |
−0.042 |
0.806 |
0.423 |
0.010 |
−0.023 |
0.892 |
|
After |
0.105 |
0.544 |
−0.123 |
0.472 |
−0.323 |
0.054 |
−0.159 |
0.353 |
0.497 |
0.002 |
0.039 |
0.820 |
DISCUSSION
The current study was conducted to evaluate the effect of synbiotic supplementation on GI function, serum levels of PTH, ALP, and other parameters in HD patients, with the aim of managing bone complications and anemia in this population. The synbiotic supplement resulted in a significant reduction in PTH levels among HD patients during the study; however, when compared to the placebo group, this difference was not statistically significant. Serum levels of ALP decreased in the synbiotic group, although the difference was not statistically significant. By the end of the study, a significant difference in ALP levels was observed between the 2 groups. However, no statistically significant differences were noted in the levels of calcium, phosphorus, albumin, hemoglobin, ferritin, TIBC, HCT, and platelet count following the consumption of the synbiotic supplement. The improvement of GI symptoms occurred in both groups. Only diarrhea in the control group had no statistically significant difference at the end of the study. There was a significant difference between the 2 groups in all symptoms except abdominal pain at the end of the study.
The results of this study indicated an improvement PTH levels in the synbiotic group following supplementation. Although the use of synbiotic supplements did not result in a significant change in ALP levels within the synbiotic group, there were meaningful differences between the 2 groups at the end of the study. No significant change was observed in calcium and phosphorus levels. Additionally, various studies have reported differing results regarding the effects of probiotics on ALP and PTH levels. In the study conducted by Simeoni et al. [
16], the probiotic supplement had no significant impact on plasma intact PTH levels, however, serum levels of calcium and iron showed significant improvement. This study utilized a probiotic containing multiple bacterial strains, and the participants were patients in the third stage of CKD. In a study conducted by Azamian et al. [
13], the consumption of synbiotic supplements did not significantly affect the serum levels of calcium and phosphorus in HD patients. Behrouz et al. [
17] found that the probiotic supplement reduced ALP compared to the control group. In contrast, Mirzaeian’s study [
18] revealed that supplementation with synbiotics for 8 week in HD patients might increase serum levels of PTH. The intervention in this study was conducted over an 8-week period and the probiotic supplement contained
Lactobacillus,
Bifidobacterium, and
Streptococcus bacterial strains. PTH is crucial in bone remodeling, as it significantly affects bone formation and absorption. The gut microbiota may modulate the effect of PTH on bone health [
19]. Activation of butyrate produced by gut microbiota enhances the anabolic activity of PTH in bone. Furthermore, the probiotic supplement
L. rhamnosus GG increases butyrate production and stimulates bone formation [
20]. Additional studies are needed to investigate the effect of probiotics on of ALP and PTH levels.
In the present study, the consumption of synbiotic supplements by HD patients had no significant effect on their serum albumin levels. However, previous research has yielded varied results. Consistent with the findings of this study, Michalickova et al. [
21] reported no significant effect of probiotic supplementation on serum albumin levels in athletes. Conversely, Zhu et al. [
22] observed a decrease in serum albumin concentration in CKD mice, but interestingly, oral gavage of probiotic supplements resulted in an increased in serum albumin levels in these mice. Most studies have been conducted in animal models, highlighting the need for further research to investigate the effects of probiotics on serum albumin levels in humans.
The data analysis of the current study did not reveal any statistically significant differences in levels of blood hemoglobin, hematocrit, platelets, ferritin, and TIBC at the end of the study. In contrast, Kooshki et al. [
23] demonstrated that synbiotic supplementation significantly improved hemoglobin and hematocrit concentrations compared to the control group while TIBC significantly decreased in the synbiotic group. The duration of this study was 8 weeks. In the study conducted by Skrypnik et al. [
24], an increase in iron absorption was observed in mice under conditions where synbiotic supplementation was combined with iron supplementation or an iron-rich diet. The effects of various probiotic strains on platelet function exhibit considerable variability. Generally,
L. rhamnosus strains have a lesser role in platelet aggregation and disease pathogenesis [
25]. Although these studies have only been conducted in vitro, the findings suggest significant implications for future in vivo study.
The present study indicates an improvement in the severity of GI symptoms in both groups at the end of the study. In the Mitrović et al. [
26] study, patients with CKD who consumed synbiotic supplements containing
Lactobacillus and
Bifidobacterium with inulin experienced a significant improvement in constipation; however, no significant effects were observed on other symptoms. Additionally, modulation of gut bacteria occurred with
Lactobacillus and
Bifidobacterium.
It is important to consider several factors when a person consumes probiotics for GI diseases, including disease type, probiotic strain, and duration of use. According to the Zhang et al.’s meta-analysis [
27], an effective combination for improving GI symptoms in patients with irritable bowel syndrome may involve probiotics from the
Bifidobacterium,
Lactobacillus, and
Saccharomyces genera, with a minimum dose of 10
9 CFU and a treatment duration of at least 4 weeks.
The data analysis of this study did not reveal any significant correlation between PTH, ALP, calcium, and phosphorus. However, the study by Takahashi et al. [
28] found a positive correlation between PTH and ALP in patients with non-nephropathic diabetes who were undergoing HD. According to previous studies, the presence of diabetes, duration of HD treatment, and sample size may influence the relationship between these serum markers [
29]. Further research is needed to investigate the effects of hyperglycemia on the interactions between bone cells.
Based on the data analysis of the study, a significant positive correlation was observed only between HbA1c and constipation at the end of the study, with no other correlations identified. Limited clinical studies have investigated the levels of AGEs in GI diseases. Previous studies have reported increased levels of AGE and oxidative stress markers in individuals with GI conditions, such as gastric cancer and inflammatory bowel diseases [
30]. Further research is needed to understand the impact of hyperglycemia on bone cell interactions and the effects related to AGEs products.
This study was a randomized controlled trial with a placebo that investigated the effects of a synbiotic supplement on GI function, PTH, ALP, and other parameters in patients undergoing HD. A key strength of this study was the high dose of probiotics in the synbiotic supplement. However, due to budget constraints, the study faced limitations, including the inability to estimate bone density and conduct fecal microbial cultures. Additionally, the sample size was insufficient to observe significant relationships between some parameters.
CONCLUSION
According to the results of this study, the consumption of a synbiotic supplement after 12 weeks led to an improvement in GI function, and serum levels of PTH in HD patients. While serum levels of ALP decreased in the synbiotic group, the difference was not statistically significant. However, by the end of the study, there was a significant difference between the 2 groups. The synbiotic supplement had no significant effect on the serum levels of other factors, including calcium, phosphorus, platelets, hematocrit, ferritin, hemoglobin, TIBC, and albumin. Additionally, no significant correlation was found between serum PTH levels, serum ALP, calcium, and phosphorus levels, and between AGEs, HbA1c, and GI disorders in patients. Future research and therapeutic strategies should focus on preventing cardiovascular, skeletal-mineral, and malnutrition diseases in CKD and HD patients.
Kermanshah University of Medical Scienceshttps://doi.org/10.13039/501100005317
4010134
NOTES
-
Funding: The present study was supported by the vice chancellor for research and technology Committee of Kermanshah University of Medical Sciences (grant number 4010134).
-
Conflict of Interest: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Azamian Y, Abdollahzad H.
Data curation: Azamian Y, Rouhani MH, Fatehi MH.
Formal analysis: Rezaeian S.
Investigation: Azamian Y, Abdollahzad H.
Methodology: Azamian Y, Abdollahzad H.
Writing - original draft: Azamian Y, Abdollahzad H.
Writing - review & editing: Azamian Y, Abdollahzad H, Rezaeian S, Rouhani MH, Fatehi MH.
ACKNOWLEDGEMENTS
We would like to express our utmost gratitude and appreciation to the research committee of Kermanshah University of Medical Sciences. Also, we are grateful for all the assistance provided by the staff of the dialysis department at Farabi Hospital in Isfahan.
REFERENCES
- 1. Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013;382:260-272.
- 2. Sesso RC, Lopes AA, Thomé FS, Lugon JR, Watanabe Y, et al. Report of the Brazilian chronic dialysis census 2012. J Bras Nefrol 2014;36:48-53.
- 3. Gordon PL, Sakkas GK, Doyle JW, Shubert T, Johansen KL. Relationship between vitamin D and muscle size and strength in patients on hemodialysis. J Ren Nutr 2007;17:397-407.
- 4. Oste L, Bervoets AR, Behets GJ, Dams G, Marijnissen RL, et al. Time-evolution and reversibility of strontium-induced osteomalacia in chronic renal failure rats. Kidney Int 2005;67:920-930.
- 5. Magnusson P, Sharp CA, Magnusson M, Risteli J, Davie MW, et al. Effect of chronic renal failure on bone turnover and bone alkaline phosphatase isoforms. Kidney Int 2001;60:257-265.
- 6. Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, et al. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 2014;39:230-237.
- 7. Parvaneh K, Jamaluddin R, Karimi G, Erfani R. Effect of probiotics supplementation on bone mineral content and bone mass density. Sci World J 2014;2014:595962.
- 8. Harahap IA, Suliburska J. Probiotics and isoflavones as a promising therapeutic for calcium status and bone health: a narrative review. Foods 2021;10:2685.
- 9. Zhao F, Guo Z, Kwok LY, Zhao Z, Wang K, et al. Bifidobacterium lactis Probio-M8 improves bone metabolism in patients with postmenopausal osteoporosis, possibly by modulating the gut microbiota. Eur J Nutr 2023;62:965-976.
- 10. Harahap IA, Moszak M, Czlapka-Matyasik M, Skrypnik K, Bogdański P, et al. Effects of daily probiotic supplementation with Lactobacillus acidophilus on calcium status, bone metabolism biomarkers, and bone mineral density in postmenopausal women: a controlled and randomized clinical study. Front Nutr 2024;11:1401920.
- 11. Vanitchanont M, Vallibhakara SA, Sophonsritsuk A, Vallibhakara O. Effects of multispecies probiotic supplementation on serum bone turnover markers in postmenopausal women with osteopenia: a randomized, double-blind, placebo-controlled trial. Nutrients 2024;16:461.
- 12. Yacoub R, Nugent M, Cai W, Nadkarni GN, Chaves LD, et al. Advanced glycation end products dietary restriction effects on bacterial gut microbiota in peritoneal dialysis patients; a randomized open label controlled trial. PLoS One 2017;12:e0184789.
- 13. Azamian Y, Abdollahzad H, Rezaeian S, Rouhani MH, Fatehi MH. The effect of synbiotic supplementation on plasma levels of advanced glycation end products and cardiovascular risk factors in hemodialysis patients: a double-blind clinical trial. Food Sci Nutr 2024;12:6864-6872.
- 14. Revicki DA, Wood M, Wiklund I, Crawley J. Reliability and validity of the gastrointestinal symptom rating scale in patients with gastroesophageal reflux disease. Qual Life Res 1998;7:75-83.
- 15. Vasheghani-Farahani A, Tahmasbi M, Asheri H, Ashraf H, Nedjat S, et al. The Persian, last 7-day, long form of the international physical activity questionnaire: translation and validation study. Asian J Sports Med 2011;2:106-116.
- 16. Simeoni M, Citraro ML, Cerantonio A, Deodato F, Provenzano M, et al. An open-label, randomized, placebo-controlled study on the effectiveness of a novel probiotics administration protocol (ProbiotiCKD) in patients with mild renal insufficiency (stage 3a of CKD). Eur J Nutr 2019;58:2145-2156.
- 17. Behrouz V, Aryaeian N, Zahedi MJ, Jazayeri S. Effects of probiotic and prebiotic supplementation on metabolic parameters, liver aminotransferases, and systemic inflammation in nonalcoholic fatty liver disease: a randomized clinical trial. J Food Sci 2020;85:3611-3617.
- 18. Mirzaeian S, Saraf-Bank S, Entezari MH, Hekmatdoost A, Feizi A, et al. Effects of synbiotic supplementation on microbiota-derived protein-bound uremic toxins, systemic inflammation, and biochemical parameters in patients on hemodialysis: a double-blind, placebo-controlled, randomized clinical trial. Nutrition 2020;73:110713.
- 19. Massy ZA, Drueke TB. Gut microbiota orchestrates PTH action in bone: role of butyrate and T cells. Kidney Int 2020;98:269-272.
- 20. Li JY, Yu M, Pal S, Tyagi AM, Dar H, et al. Parathyroid hormone-dependent bone formation requires butyrate production by intestinal microbiota. J Clin Invest 2020;130:1767-1781.
- 21. Michalickova D, Kotur-Stevuljevic J, Miljkovic M, Dikic N, Kostic-Vucicevic M, et al. Effects of probiotic supplementation on selected parameters of blood prooxidant-antioxidant balance in elite athletes: a double-blind randomized placebo-controlled study. J Hum Kinet 2018;64:111-122.
- 22. Zhu L, Niu J, Tang XC, Shan LH, Xiao L, et al. The effects of probiotic Lactobacillus rhamnosus GG on fecal flora and serum markers of renal injury in mice with chronic kidney disease. Front Biosci (Landmark Ed) 2023;28:226.
- 23. Kooshki A, Akbarzadeh R, Amin B, Tofighiyan T, Foroumandi E. Synbiotic supplement for treatment of iron deficiency anaemia in haemodialysis patients: a randomized controlled trial. Nephrology (Carlton) 2023;28:234-239.
- 24. Skrypnik K, Bogdański P, Sobieska M, Schmidt M, Suliburska J. Influence of multistrain probiotic and iron supplementation on iron status in rats. J Trace Elem Med Biol 2021;68:126849.
- 25. Zhou JS, Rutherfurd KJ, Gill HS. Inability of probiotic bacterial strains Lactobacillus rhamnosus HN001 and Bifidobacterium lactis HN019 to induce human platelet aggregation in vitro. J Food Prot 2005;68:2459-2464.
- 26. Mitrović M, Stanković-Popović V, Tolinački M, Golić N, Soković Bajić S, et al. The impact of synbiotic treatment on the levels of gut-derived uremic toxins, inflammation, and gut microbiome of chronic kidney disease patients-a randomized trial. J Ren Nutr 2023;33:278-288.
- 27. Zhang WX, Shi LB, Zhou MS, Wu J, Shi HY. Efficacy of probiotics, prebiotics and synbiotics in irritable bowel syndrome: a systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials. J Med Microbiol 2023;72:001758.
- 28. Takahashi N, Kojima T, Ogawa H, Ishiguro N. Correlation between parathyroid hormone, bone alkaline phosphatase and N-telopeptide of type 1 collagen in diabetic and non-diabetic haemodialysis patients. Nephrology (Carlton) 2007;12:539-545.
- 29. Inaba M, Nishizawa Y, Mita K, Kumeda Y, Emoto M, et al. Poor glycemic control impairs the response of biochemical parameters of bone formation and resorption to exogenous 1,25-dihydroxyvitamin D3 in patients with type 2 diabetes. Osteoporos Int 1999;9:525-531.
- 30. Farhadi A, Fields J, Banan A, Keshavarzian A. Reactive oxygen species: are they involved in the pathogenesis of GERD, Barrett’s esophagus, and the latter’s progression toward esophageal cancer? Am J Gastroenterol 2002;97:22-26.
 , Hadi Abdollahzad2,3
, Hadi Abdollahzad2,3 , Shahab Rezaeian4
, Shahab Rezaeian4 , Mohammad Hossein Rouhani5
, Mohammad Hossein Rouhani5 , Mohammad Hossein Fatehi6
, Mohammad Hossein Fatehi6