ABSTRACT
An insufficient intake of magnesium may be associated with the development of chronic obstructive pulmonary disease (COPD). We aimed to determine the relationship between health related quality of life (QoL), anthropometric indices and nutritional status with dietary magnesium intake in COPD patients. Sixty-one COPD patients participated in this cross-sectional study. QoL and nutritional status were assessed. Furthermore, body composition, calf circumference, and muscle strength were measured; equations were used to calculate fat-free mass index, body mass index, and muscle mass value. Dietary magnesium intake was assessed by three 24-hours recalls and magnesium intake was categorized as ≤ 188.08 mg/day (A group) and > 188.08 mg/day (B group). The χ2, independent-sample t-test and Mann-Whitney test were used for statistical analysis. The p values less than 0.05 were considered significant. Of QoL assessments the total and impact mean scores of St. George's respiratory questionnaire in the B group were significantly lower than the means of the A group (p value = 0.007 and 0.005, respectively). The instrumental activity of daily living score was significantly improved in patients with higher consumption of dietary magnesium (p = 0.02). Participants had a significantly lower mean score of patient-generated subjective global assessment in the B group compared to the A group (p = 0.003). Higher intake of dietary magnesium can lead to improve QoL and nutrition status.
-
Keywords: Body composition; Magnesium; Nutritional status; Quality of life; Pulmonary disease, chronic obstructive
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a progressive respiratory disorder characterized by lung function impairment [
1,
2,
3] and is a fourth-leading cause of mortality, which expected to cause over 6 million death worldwide per year by 2030 [
4,
5]. COPD patients may suffer from muscle weakness, osteoporosis, diabetes, cardiovascular disease, hypertension, depression, and lung cancer [
6]. The loss of working ability or early retirement caused by physical disability can result in substantial socioeconomic losses and health expenditures in COPD patients [
7].
The most common clinical sign of this disease is airway obstruction, which mainly leads to a lowered quality of life (QoL), as it limits exercise capability, hinders social interaction, and creates mental symptoms like anxiety and depression [
8]. Other main factors that are associated with QoL in COPD patients include age, gender, psychosocial status, body mass index (BMI), and comorbid conditions [
8]. Furthermore, the risk of readmission to the hospital is higher for people with moderate to severe COPD and poor QoL, and they are more likely to need a nebulizer at home [
9].
Nutritional abnormalities are another adverse outcome standing out among systemic manifestations in patients with chronic respiratory conditions. Approximately 30%–60% of people with COPD are malnourished, which is largely caused by a high metabolic rate and an inadequate nutritional intake; patients with COPD have a 10 times greater breath-related energy expenditure each day than they would normally require, which may result in malnutrition if they are not compensated by proportional energy intake [
10]. Therefore, diagnosis of patients with impaired nutritional status is of paramount importance when deciding on the appropriate therapeutic approach [
11].
A recent study has suggested that insufficient intake of magnesium may be associated with the development of asthma or COPD [
12]. Studies have shown that patients with COPD and those over the age of 65 tend to have a reduced level of magnesium in their bodies [
13,
14]. In addition to its role in bronchodilation, contraction in smooth muscles of the respiratory tract, and the release of neurohumoral mediators, magnesium is believed to have a protective effect against chronic respiratory tract diseases [
12]. Moreover, for the sake of its ability to block calcium channels and suppress acetylcholine release, magnesium plays a critical role in muscle strength and exercise performance [
15,
16,
17]. The lack of magnesium is often overlooked, as it is not detected with a simple serum magnesium dosage [
18]. Consequently, magnesium deficiency can lead to bronchoconstriction, lowered physical performance [
17], and exacerbation of disease [
13].
Despite the importance of the role of magnesium in respiratory diseases, we are aware of a few publications in the literature regarding this area; hence, the aim of the present study was to evaluate the associations between dietary magnesium intake and QoL, anthropometric indices, muscle strength, and nutritional status in COPD patients.
MATERIALS AND METHODS
Study design and participants
This cross-sectional study was performed from September to December 2018 in participants with COPD, selected from the patients with medical records from 4 medical centers related to Shiraz University of Medical Sciences, Shiraz, Iran (Rajai, Nemazee, Shahid Faghihi, and Ali-Asghar hospitals) between March 2011 to June 2018 and specialized respiratory clinics. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures were approved by the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran; IR.SUMS.REC.1396.85. Moreover, written informed consent was obtained from all subjects.
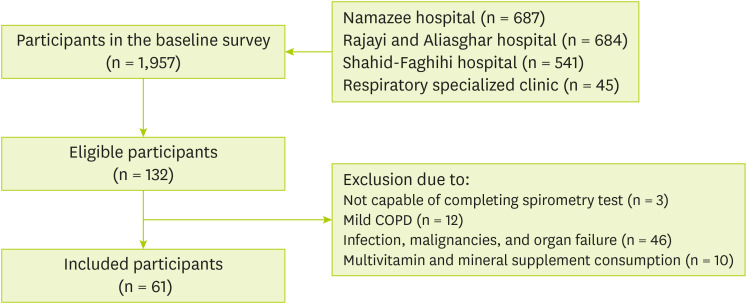
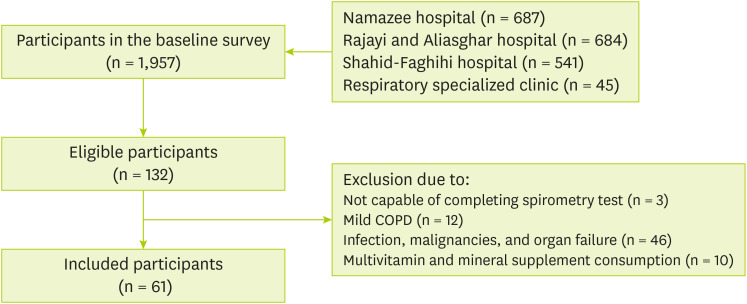
The census method was used to screen a total of 1,957 patients for participation. One hundred and twenty nine out of 132 volunteer patients completed the spirometry test. Finally, only 61 patients were eligible and included to participate in our study (
Figure 1).
Figure 1
Flow chart of the study.
COPD, chronic obstructive pulmonary disease.
Inclusion and exclusion criteria
In this study, the inclusion criteria were men aged 40–70 years with moderate and severe severity of COPD for 7 years and diagnosed for at least 3 months who resided in Shiraz. While women and individuals with mild severity of COPD, infections, malignancies, other inflammatory diseases, any other organ failure, and individuals using supplements were not eligible for this study.
Procedure
Initially, through calling and asking several queries, patients who were willing to participate in the study were referred to Imam Reza Clinic (Clinic affiliated to Shiraz University of Medical Sciences). In addition, an educated person based on the Global Initiative for Chronic Obstructive Lung Disease (GOLD) committee checked and reevaluated the records of individuals diagnosed with COPD through obstructive pulmonary spirometry. The forced expiratory volume in 1 second (FEV1) to forced vital capacity ratio < 0.70 value and fixed FEV1 values were used for anticipation of the disease and categorize its severity which previously explained in details [
19]. Also, a pulmonary specialist reaffirmed patients with COPD again. Then, the aim of the present experiment was clarified to the patients and written consent was gained. Finally, socio-demographic information, anthropometric indices, body composition, muscle strength, nutritional status, and health-related QoL (HRQoL) questionnaires were gathered for each participant by the researcher.
Height was measured using a stadiometer to the nearest 0.1 cm. Body weight was measured to the nearest 0.1 kg using (Omron, Korea) scale while participants were in light clothes. Standard methods were considered to measure the calf circumference (CC) by a non-stretchable measuring tape to the nearest 0.1 cm in a seated position [
20]. Body composition indices, including lean body mass, body fat mass, arm muscle circumference (AMC), and fat-free mass (FFM) were determined by bioelectrical impedance analysis (BIA) and InBody S10 analyzer (Bio-Space Co., Ltd., Seoul, Korea) [
20]. Dividing segmental lean body mass to height squared (m
2) and FFM to height squared (m
2) were used for calculating muscle mass values [
21] and FFM index (FFMI) [
22], respectively.
Muscular strength was measured in seated position 3 times for each hand by hydraulic hand dynamometer (model MSD; Saehan, Changwon, Korea) as a simple tool. Both hands were measured 3 times to calculate the mean grip strength [
23]. Body position was fixed considering to the device working protocol.
To assess nutrition-related complications, the primary investigator used the patient-generated subjective global assessment (PG-SGA) questionnaire, which was based on the patient's medical history (Body weight, % weight loss, gastrointestinal symptoms, dietary intake, functional capacity, and respiratory stress) and physical examination (subcutaneous fat loss, muscle wasting and edema) [
24]. Furthermore, the mean of three 24-hour dietary recalls (involving 2 weekdays and one weekend day) was used to evaluate the dietary intake of magnesium (≤ 188.08 mg/day and > 188.08 mg/ day based on the 50th percentile); also, participants were asked about changes in their diet and they noted that no changes were made to their diet due to conducting the study over a period of one season (autumn). Nutrient composition was assessed by the Nutritionist IV software version 3.5.2 (Hearst Corp., San Bruno, SA, USA) modified based on Iranian foods [
25].
A variety of questionnaires were used to assess the QoL based on lung function, such as the St. George's respiratory questionnaire (SGRQ) which consists of 14 items categorized into symptoms (frequency and severity of respiratory), activity (role limitations as a result of breathlessness), and impact (psychological and social functioning disturbances because of respiratory disorder), instrumental activities of daily living (IADLs) which using to assess the ability to perform daily tasks independently and Katz Index to determine dependency status in activities of daily living, described by details previously [
26].
We analyzed the data using SPSS software version 21.0 (SPSS Inc., Chicago, IL, USA). Normality of the data was assessed by Kolmogorove-Smirnov test. The Chi-square was used for qualitative statistical data. Comparison between 2 groups with normal and abnormal distribution was determined by independent-sample t-test and Mann-Whitney U test, respectively. A p value less than 0.05 was considered significant.
RESULTS
Table 1 shows participant's characteristics based on classification of dietary magnesium intake. From now on, the patients receiving magnesium ≤ 188.08 mg/day are called A group and the participants receiving magnesium > 188.08 mg/day are called B group.
Table 1 Characteristics of the participants based on classification of dietary magnesium intake
Table 1
|
Variables |
Magnesium intake (mg/day) |
Variables classification |
No. (%) |
p value*
|
|
Age (yr) |
A group |
40–65 |
18 (29.50) |
0.41 |
|
> 65 |
13 (21.32) |
|
B group |
40–65 |
14 (22.95) |
|
> 65 |
16 (26.23) |
|
Education |
A group |
Illiterate |
7 (11.47) |
0.34 |
|
Elementary |
13 (21.32) |
|
≥ Intermediate |
11 (18.04) |
|
B group |
Illiterate |
4 (6.55) |
|
Elementary |
16 (26.22) |
|
≥ Intermediate |
10 (16.40) |
|
Job exposure |
A group |
Yes |
27 (44.27) |
0.45 |
|
No |
4 (6.55) |
|
B group |
Yes |
24 (39.34) |
|
No |
6 (9.84) |
|
Smoking status |
A group |
Yes |
20 (32.78) |
0.16 |
|
No |
11 (18.4) |
|
B group |
Yes |
14 (22.95) |
|
No |
16 (26.23) |
|
Age at onset of smoking (yr) |
A group |
13–25 |
20 (37.04) |
0.50 |
|
26–59 |
8 (14.81) |
|
B group |
13–25 |
15 (27.77) |
|
26–59 |
11 (20.38) |
|
Years of smoking |
A group |
3–35 |
10 (18.18) |
0.52 |
|
36–60 |
18 (32.73) |
|
B group |
3–35 |
14 (25.45) |
|
36–60 |
13 (23.64) |
|
Energy (kcal/day)†
|
A group |
1,221.92 ± 416.57 |
- |
0.22 |
|
B group |
1,349.04 ± 396.24 |
- |
|
Protein (g/day)‡
|
A group |
50.39 ± 26.07 |
- |
0.07 |
|
B group |
52.29 ± 11.29 |
- |
|
Vitamin C (mg/day)†
|
A group |
103.08 ± 86.75 |
- |
0.16 |
|
B group |
136.46 ± 98.59 |
- |
Less than 23% of patients in the B group were between 45–65 years old, while this was about 30% in the A group. In the A group, approximately 33% of the participants smoked and about 18% of them never smoked, although this proportion was about 23% and 26%, respectively, in the B group. Furthermore, the mean intake of energy, protein and vitamin C was higher in the B group than group A. None of the other variables were significant difference between the 2 A and B groups.
Table 2 reports the mean score of participants' QoL in the A and B groups. Among the questionnaire tools related to QoL assessment, results revealed that in the B group, the total and impact mean scores of SGRQ were significantly lower than their means in the A group (p = 0.007 and 0.005 respectively). The means of SGRQ activity and symptom scores in the B group were lower than the A group, though this did not meet statistical significance (p = 0.15 and 0.43, respectively). Furthermore, contrary to the A group, the IADL mean score was significantly higher in the B group (p = 0.02). Due to the Katz index, its mean score was higher in the B group when compared to the A group, while the difference was not significant (p = 0.21).
Table 2 Correlation between health-related quality of life according to SGRQ scores; Katz index and IADL score with dietary magnesium intake
Table 2
|
Variables |
Magnesium intake (mg/day) |
Mean ± SD |
Median (IQR) |
p value*
|
|
SGRQ (total score)‡
|
A group |
67 ± 14 |
74 (61–78) |
0.007*
|
|
B group |
57 ± 15 |
58 (43–70) |
|
SGRQ (impact score)‡
|
A group |
63 ± 17 |
69 (52–74) |
0.005*
|
|
B group |
51 ± 18 |
56 (38–63) |
|
SGRQ (activity score)‡
|
A group |
71 ± 18 |
75 (59–84) |
0.15 |
|
B group |
64 ± 20 |
67 (52–78) |
|
SGRQ (symptom score)†
|
A group |
74 ± 15 |
74 (64–86) |
0.43 |
|
B group |
62 ± 13 |
61 (53–72) |
|
Katz index‡
|
A group |
5.35 ± 0.95 |
6 (5–6) |
0.21 |
|
B group |
5.56 ± 0.93 |
6 (5–6) |
|
IADL score‡
|
A group |
4.64 ± 1.76 |
5 (3–6) |
0.02*
|
|
B group |
5.60 ± 0.67 |
6 (5–6) |
Comparison of anthropometric indices, body composition, muscle strength and nutritional status in the 2 A and B groups are illustrated in
Table 3. Results indicated that BMI, muscle mass value, protein BIA, FFM, arm circumference, AMC, and CC have higher mean in the B group, however, these differences were not statistically significant in comparison to the A group (p > 0.05 for all). In terms of muscle strength, both mean of right and left handgrip in the B group were insignificantly lower than the A group (p = 0.69 and 0.30, respectively).
Table 3 Correlation between anthropometric indices, body composition, muscle strength and nutritional status with magnesium intake
Table 3
|
Variables |
Magnesium intake (mg/day) |
Mean ± SD |
Median (IQR) |
p value*
|
|
BMI (kg/m2)†
|
A group |
20.13 ± 2.46 |
20.20 (18.20–21.70) |
0.19 |
|
B group |
21.94 ± 3.05 |
23.05 (20.10–23.70) |
|
Muscle mass value (kg)†
|
A group |
6.76 ± 0.78 |
6.74 (5.98–7.32) |
0.10 |
|
B group |
6.96 ± 0.62 |
6.95 (6.71–7.28) |
|
Protein BIA†
|
A group |
9.12 ± 1.23 |
9.10 (7.90–9.80) |
0.17 |
|
B group |
9.30 ± 1.05 |
9.25 (8.77–9.80) |
|
Fat-free mass (kg)†
|
A group |
46.41 ± 6.02 |
46.20 (40.80–49.80) |
0.23 |
|
B group |
47.37 ± 5.30 |
47.15 (44.65–49.72) |
|
FFMI (kg/m2)†
|
A group |
16.35 ± 1.47 |
16.73 (15.50–17.43) |
0.35 |
|
B group |
16.95 ± 1.45 |
17.05 (16.32–17.91) |
|
Arm circumference†
|
A group |
28.04 ± 2.46 |
27.50 (26.70–30.20) |
0.55 |
|
B group |
29.15 ± 2.85 |
29.20 (27.62–31.15) |
|
AMC†
|
A group |
23.83 ± 2.01 |
24.20 (22.60–25.10) |
0.76 |
|
B group |
24.09 ± 1.81 |
24.55 (22.80–25.25) |
|
Calf circumference (cm)†
|
A group |
30.54 ± 2.15 |
30 (29–31) |
0.44 |
|
B group |
32.13 ± 2.53 |
32.50 (30.75–34) |
|
Mean handgrip right (kg)‡
|
A group |
22.67 ± 6.16 |
21.33 (18.66–24) |
0.69 |
|
B group |
22.31 ± 6.42 |
20.66 (17.33–26) |
|
Mean handgrip left (kg)‡
|
A group |
22.97 ± 8.20 |
21.66 (17.16–28.16) |
0.30 |
|
B group |
20.85 ± 6.33 |
19.33 (16–24.66) |
|
PG-SGA score‡
|
A group |
12.96 ± 6.65 |
12 (9–15) |
0.003*
|
|
B group |
8.93 ± 3.70 |
8 (6–12) |
Considering nutritional status, participants had a significantly lower mean score of PG-SGA in the B group as compared to the A group (p = 0.003).
DISCUSSION
The results of this cross-sectional study revealed that the higher dietary intake of magnesium could improve HRQoL scores including total and impact scores of SGRQ and IADL score and nutritional status as well.
HRQoL is a valuable means measuring the impact of ailments on an individual’s well-being and their daily life. As a multidimensional concept, it considers 3 domains related to the physical, social, and psychological impact of the disease [
27]. Poor HRQoL in COPD patients is negatively linked to physical impairment, and depression, as well as an increased risk of exacerbations and mortality [
9].
Our results revealed that higher consumption of dietary magnesium may significantly improve the total and impact (disturbances in psychological and social functioning caused by respiratory disorders) scores of SGRQ, as well as, the IADL score which used for evaluating the ability to handle routine tasks independently.
There are few data on magnesium's effect on QoL in patients with pulmonary diseases, despite its importance in human physiology [
28]. Some clinical trials examine the effects of magnesium supplementation on QoL, but no studies have examined the relationship between dietary magnesium and QoL. Kazaks et al. [
29], found that magnesium supplementation (170 mg twice a day for 6.5 months) has a beneficial effect on improving QoL score in patients with mild to moderate asthma [
29]. Furthermore, significant improvement in both mental and physical aspects of QoL was seen in the Noah et al. [
28] study who investigates the effect of magnesium with a daily dose 300 mg during 8 weeks in stressed healthy adults. Regarding to available evidence, the positive effect of magnesium intake on COPD-related QoL through psychological improvements could be related to the neuroprotective effect of magnesium [
30]. However, Zanforlini et al. [
31], in their study showed that 300 mg/day magnesium did not have any significant effect on QoL in patients with COPD. Likewise, another study showed non-significant effect of magnesium (340 mg per day for 2 months) on QoL of patients with mild to moderate asthma [
32].
Body composition is complex to determine in COPD patients; their loss of muscle mass may occur with or without accompanying changes in fat mass, resulting in underweight or obese condition with underlying muscle dysfunction [
33]. The higher the body weight of COPD patients, the better their prognosis is, but excess body fat can also pose additional health problems [
34].
We found that BMI, muscle mass value, protein BIA, FFM, FFMI, arm circumference, AMC, and CC were higher in individuals with greater intake of dietary magnesium (> 188.08 mg/day), although, these differences were not statistically significant in comparison to the A group. In addition, higher magnesium consumption was not significantly correlated with muscle strength.
Studies investigating the relationship of magnesium dietary intake/magnesium supplement with anthropometric indices, have been done in different population and there is inconsistency in their results. In a study conducted by Moslehi et al. [
35], the efficacy of 250 mg of magnesium in older age women was investigated and their results showed that in comparison to baseline values, there were significant increases in lean body mass, improvement in handgrip strength, and exclusive declines in fat mass among the magnesium group at the end of 8 weeks, but there was no significant difference compared to control groups. According to Welch et al. [
36], indices of skeletal muscle and leg explosive power were correlated positively with dietary magnesium. Another study observed the association between the dietary intake of nutrients and sarcopenia among older adults; the fully adjusted model revealed that sarcopenic compared with non-sarcopenic individuals consumed slightly lower amounts of potassium, magnesium, phosphorus, iron, and vitamin K [
37]. However, the study of Gohari-Kahou et al. [
38], revealed no association between levels of serum and dietary magnesium with abdominal obesity amongst 853 Iranian adults with metabolic syndrome. When it comes to muscle strength, a cross-sectional study conducted by Gedmantaite et al. [
39], demonstrated the positive association between magnesium intake and handgrip strength in both sexes. Similarly, Setaro et al. [
40], showed the significant association between dietary magnesium intake and muscle strength in twenty-six male athletes. Dominguez et al. [
41], reported that the total serum magnesium was in positive correlation with various measurements of muscle strength, in particular; grip strength. Furthermore, some studies showed that the non-sarcopenia subjects had a significantly higher intake of dietary magnesium when compared to the sarcopenic subjects [
42,
43].
Magnesium plays a key role in energy metabolism, transmembrane transport, muscle contraction, and relaxation, which may explain why it strongly influences muscle performance [
44]. Also, it modulates anabolic hormones secretion specifically testosterone and insulin-like growth factor-1, since it can act as an uncompetitive inhibitor to testosterone binding in biological concentrations and consequently increase free-testosterone within the body [
45]. Additionally, magnesium has a pivotal role in calcium and potassium ions exchanging across cell membranes, as an essential contribution to neuronal activity and muscle contractions [
46]. Magnesium depletion, leading to increase in oxidative stress and impairment of intracellular calcium homeostasis, can damage muscle cells [
41]. Furthermore, magnesium supports basic mitochondrial processes, including adenosine triphosphate synthesis, electron transport chain complex subunits, and oxygen detoxification contributing to provide energy [
47,
48]. Insufficient magnesium availability, therefore, is likely to cause mitochondrial inefficiency and elevated reactive oxygen species production, thereby affecting protein structure and function [
41]. Thus, it is likely that magnesium can influence muscle strength and function in the long run via these mechanisms.
With prevalence rates reported between 20% and 45%, malnutrition in patients with COPD is associated with an increased risk of complications, longer hospital stays, decreased QoL, and death, resulting in an increased health care economic cost [
49,
50,
51]. COPD patients with an imbalance between their energy requirements and energy expenditure might suffer from weight loss and muscle depletion, or even be worse off by death [
49,
51]. We found that a greater intake of dietary magnesium was associated in a helpful direction with PG-SGA score significantly. Fein et al. [
52], observed that serum magnesium levels were straightly associated with serum markers of nutrition, including: albumin, creatinine, and total protein; an inverse correlation was seen between serum magnesium and the extracellular mass/body cell mass ratio, a highly sensitive marker of malnutrition [
51].
Our study had some limitations; first, casual relationships could not be determined because of its cross-sectional design; second, despite the fact that some women suffered from COPD and its complications, they could not be included because there were not enough women who qualified for the study. In other words, the inclusion of a few women might change the balance of gender distribution in the study, which could impact interpretation of the results; further nutrition research in COPD would be beneficial to gain insight into what influences the progression and the management of the disease.
CONCLUSION
Our results revealed that higher dietary intake of magnesium can improve QoL and nutritional status in COPD patients. Future studies in COPD are suggested to answer the questions and inconclusive results.
Shiraz University of Medical Scienceshttps://doi.org/10.13039/501100004320
13723
NOTES
-
Funding: This research was supported by grants from Shiraz University of Medical Sciences (grant No.13723).
-
Conflict of Interest: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Ahmadi A.
Data curation: Ahmadi A.
Formal analysis: Zare M.
Funding acquisition: Eftekhari MH.
Investigation: Ahmadi A.
Methodology: Fararooei M and Mazloom Z.
Project administration: Eftekhari MH.
Supervision: Eftekhari MH.
Writing - original draft: Ahmadi A.
Writing - review & editing: Ahmadi A, Eftekhari MH, Mazloom Z, Masoompour M, Fararooei M, Zare M, and Hejazi N.
ACKNOWLEDGEMENTS
The authors thank the cooperation of the participating patients. This study was extracted from Afsane Ahmadi's PhD dissertation.
REFERENCES
- 1. Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, Menezes AM, Sullivan SD, Lee TA, Weiss KB, Jensen RL, Marks GB, Gulsvik A, Nizankowska-Mogilnicka E. BOLD Collaborative Research Group. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007;370:741-750.
- 2. Ko FWS, Hui DSC, Lai CKW. Worldwide burden of COPD in high- and low-income countries. Part III. Asia-Pacific studies. Int J Tuberc Lung Dis 2008;12:713-717.
- 3. Yang K, Wu Y, Chen D, Liu S, Chen R. The impact of lung function on extra-pulmonary diseases and all-cause mortality in US adult population with and without COPD. Clin Epidemiol 2020;12:997-1005.
- 4. Onishi K. Total management of chronic obstructive pulmonary disease (COPD) as an independent risk factor for cardiovascular disease. J Cardiol 2017;70:128-134.
- 5. World Health Organization. Chronic respiratory diseases, burden of chronic obstructive pulmonary disease. Geneva: World Health Organization; 2015.
- 6. Choi HS, Yang DW, Rhee CK, Yoon HK, Lee JH, Lim SY, Kim YI, Yoo KH, Hwang YI, Lee SH, Park YB. The health-related quality-of-life of chronic obstructive pulmonary disease patients and disease-related indirect burdens. Korean J Intern Med (Korean Assoc Intern Med) 2020;35:1136-1144.
- 7. Lou P, Zhu Y, Chen P, Zhang P, Yu J, Zhang N, Chen N, Zhang L, Wu H, Zhao J. Vulnerability, beliefs, treatments and economic burden of chronic obstructive pulmonary disease in rural areas in China: a cross-sectional study. BMC Public Health 2012;12:287.
- 8. Xiao T, Wu K, Chen Y, Qiu H, Ruan X, Wang N, Zhao Q, Fu C. Quality of life and its associated factors for mild chronic obstructive pulmonary disease patients of urban community settings. Ann Palliat Med 2020;9:1420-1430.
- 9. Long H, Howells K, Peters S, Blakemore A. Does health coaching improve health-related quality of life and reduce hospital admissions in people with chronic obstructive pulmonary disease? A systematic review and meta-analysis. Br J Health Psychol 2019;24:515-546.
- 10. Hancu A. Nutritional status as a risk factor in COPD. Maedica (Bucur) 2019;14:140-143.
- 11. Gea J, Sancho-Muñoz A, Chalela R. Nutritional status and muscle dysfunction in chronic respiratory diseases: stable phase versus acute exacerbations. J Thorac Dis 2018;10:S1332-S1354.
- 12. Gumus A, Haziroglu M, Gunes Y. Association of serum magnesium levels with frequency of acute exacerbations in chronic obstructive pulmonary disease: a prospective study. Pulm Med 2014;2014:329476.
- 13. Barbagallo M, Gupta RK, Dominguez LJ, Resnick LM. Cellular ionic alterations with age: relation to hypertension and diabetes. J Am Geriatr Soc 2000;48:1111-1116.
- 14. Veronese N, Zanforlini BM, Manzato E, Sergi G. Magnesium and healthy aging. Magnes Res 2015;28:112-115.
- 15. Corrêa F, Farah CS, Salinas RK. Mg2+ ions bind at the C-terminal region of skeletal muscle α-tropomyosin. Biopolymers 2009;91:583-590.
- 16. Grabarek Z. Insights into modulation of calcium signaling by magnesium in calmodulin, troponin C and related EF-hand proteins. Biochim Biophys Acta 2011;1813:913-921.
- 17. Ye M, Li Q, Xiao L, Zheng Z. Serum magnesium and fractional exhaled nitric oxide in relation to the severity in asthma-chronic obstructive pulmonary disease overlap. Biol Trace Elem Res 2021;199:1771-1777.
- 18. Barbagallo M, Belvedere M, Dominguez LJ. Magnesium homeostasis and aging. Magnes Res 2009;22:235-246.
- 19. Ahmadi A, Mazloom Z, Eftekhari MH, Masoompour SM, Fararouei M, Eskandari MH, Mehrabi S, Zare M, Sohrabi Z. Muscle mass and function are related to respiratory function in chronic obstructive pulmonary disease. Med J Islam Repub Iran 2021;35:34.
- 20. Ramachandran K, McCusker C, Connors M, Zuwallack R, Lahiri B. The influence of obesity on pulmonary rehabilitation outcomes in patients with COPD. Chron Respir Dis 2008;5:205-209.
- 21. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95-101.
- 22. Schols AM, Ferreira IM, Franssen FM, Gosker HR, Janssens W, Muscaritoli M, Pison C, Rutten-van Mölken M, Slinde F, Steiner MC, Tkacova R, Singh SJ. Nutritional assessment and therapy in COPD: a European Respiratory Society statement. Eur Respir J 2014;44:1504-1520.
- 23. Massy-Westropp NM, Gill TK, Taylor AW, Bohannon RW, Hill CL. Hand grip strength: age and gender stratified normative data in a population-based study. BMC Res Notes 2011;4:127.
- 24. Bauer J, Egan E, Clavarino A. The scored patient-generated subjective global assessment is an effective nutrition assessment tool in subjects with chronic obstructive pulmonary disease. E Spen Eur E J Clin Nutr Metab 2011;6:e27-e30.
- 25. Schröder H, Covas MI, Marrugat J, Vila J, Pena A, Alcántara M, Masiá R. Use of a three-day estimated food record, a 72-hour recall and a food-frequency questionnaire for dietary assessment in a Mediterranean Spanish population. Clin Nutr 2001;20:429-437.
- 26. Ahmadi A, Eftekhari MH, Mazloom Z, Masoompour M, Fararooei M, Eskandari MH, Mehrabi S, Bedeltavana A, Famouri M, Zare M, Nasimi N, Sohrabi Z. Fortified whey beverage for improving muscle mass in chronic obstructive pulmonary disease: a single-blind, randomized clinical trial. Respir Res 2020;21:216.
- 27. Bakas T, McLennon SM, Carpenter JS, Buelow JM, Otte JL, Hanna KM, Ellett ML, Hadler KA, Welch JL. Systematic review of health-related quality of life models. Health Qual Life Outcomes 2012;10:134.
- 28. Noah L, Dye L, Bois De Fer B, Mazur A, Pickering G, Pouteau E. Effect of magnesium and vitamin B6 supplementation on mental health and quality of life in stressed healthy adults: post-hoc analysis of a randomised controlled trial. Stress Health 2021;37:1000-1009.
- 29. Kazaks AG, Uriu-Adams JY, Albertson TE, Shenoy SF, Stern JS. Effect of oral magnesium supplementation on measures of airway resistance and subjective assessment of asthma control and quality of life in men and women with mild to moderate asthma: a randomized placebo controlled trial. J Asthma 2010;47:83-92.
- 30. Al Alawi AM, Majoni SW, Falhammar H. Magnesium and human health: perspectives and research directions. Int J Endocrinol 2018;2018:9041694.
- 31. Zanforlini BM, Ceolin C, Trevisan C, Alessi A, Seccia DM, Noale M, Maggi S, Guarnieri G, Vianello A, Sergi G. Clinical trial on the effects of oral magnesium supplementation in stable-phase COPD patients. Aging Clin Exp Res 2021.
- 32. Hosseini SA, Fathi N, Tavakkol H, Yadollahpour A. Investigating the effects of oral magnesium citrate supplement on lung function, magnesium level and interlukine-17 in patients with asthma. Int J Pharm Res Allied Sci 2016;5:86-92.
- 33. Schols A, Broekhuizen R, Scheepers CW, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease: Am J Clin Nutr 2005 Jul;82(1):53–9. Respir Medicine COPD Update 2005;1:69-70.
- 34. Passey SL, Hansen MJ, Bozinovski S, McDonald CF, Holland AE, Vlahos R. Emerging therapies for the treatment of skeletal muscle wasting in chronic obstructive pulmonary disease. Pharmacol Ther 2016;166:56-70.
- 35. Moslehi N, Vafa M, Sarrafzadeh J, Rahimi-Foroushani A. Does magnesium supplementation improve body composition and muscle strength in middle-aged overweight women? A double-blind, placebo-controlled, randomized clinical trial. Biol Trace Elem Res 2013;153:111-118.
- 36. Welch AA, Kelaiditi E, Jennings A, Steves CJ, Spector TD, MacGregor A. Dietary magnesium is positively associated with skeletal muscle power and indices of muscle mass and may attenuate the association between circulating C-reactive protein and muscle mass in women. J Bone Miner Res 2016;31:317-325.
- 37. Beaudart C, Locquet M, Touvier M, Reginster JY, Bruyère O. Association between dietary nutrient intake and sarcopenia in the SarcoPhAge study. Aging Clin Exp Res 2019;31:815-824.
- 38. Gohari-Kahou M, Darroudi S, Saberi-Karimian M, Parizadeh SM, Asadi Z, Javandoost A, Safarian M, Mouhebati M, Ebrahimi M, Ferns GA, Kazerani HR, Ghayour-Mobarhan M. The association between serum and dietary magnesium with cardiovascular disease risk factors in Iranian adults with metabolic syndrome. Transl Metab Syndr Res 2020;3:42-48.
- 39. Gedmantaite A, Celis-Morales CA, Ho F, Pell JP, Ratkevicius A, Gray SR. Associations between diet and handgrip strength: a cross-sectional study from UK Biobank. Mech Ageing Dev 2020;189:111269.
- 40. Setaro L, Santos-Silva PR, Nakano EY, Sales CH, Nunes N, Greve JM, Colli C. Magnesium status and the physical performance of volleyball players: effects of magnesium supplementation. J Sports Sci 2014;32:438-445.
- 41. Dominguez LJ, Barbagallo M, Lauretani F, Bandinelli S, Bos A, Corsi AM, Simonsick EM, Ferrucci L. Magnesium and muscle performance in older persons: the InCHIANTI study. Am J Clin Nutr 2006;84:419-426.
- 42. Ter Borg S, de Groot LC, Mijnarends DM, de Vries JH, Verlaan S, Meijboom S, Luiking YC, Schols JM. Differences in nutrient intake and biochemical nutrient status between sarcopenic and nonsarcopenic older adults-results from the Maastricht Sarcopenia Study. J Am Med Dir Assoc 2016;17:393-401.
- 43. Verlaan S, Aspray TJ, Bauer JM, Cederholm T, Hemsworth J, Hill TR, McPhee JS, Piasecki M, Seal C, Sieber CC, Ter Borg S, Wijers SL, Brandt K. Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: a case-control study. Clin Nutr 2017;36:267-274.
- 44. Lukaski HC. Vitamin and mineral status: effects on physical performance. Nutrition 2004;20:632-644.
- 45. Kheyruri F, Sarrafzadeh J, Hosseini AF, Abiri B, Vafa M. Randomized study of the effects of vitamin D and magnesium Co-supplementation on Muscle strength and function, body composition, and inflammation in vitamin D-deficient middle-aged women. Biol Trace Elem Res 2021;199:2523-2534.
- 46. Erem S, Atfi A, Razzaque MS. Anabolic effects of vitamin D and magnesium in aging bone. J Steroid Biochem Mol Biol 2019;193:105400.
- 47. Wolf FI, Cittadini A. Chemistry and biochemistry of magnesium. Mol Aspects Med 2003;24:3-9.
- 48. Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A 2005;102:5618-5623.
- 49. Lim S, Lam DC, Muttalif AR, Yunus F, Wongtim S, Lan TT, Shetty V, Chu R, Zheng J, Perng DW, de Guia T. Impact of chronic obstructive pulmonary disease (COPD) in the Asia-Pacific region: the EPIC Asia population-based survey. Asia Pac Fam Med 2015;14:4.
- 50. Ingadottir AR, Beck AM, Baldwin C, Weekes CE, Geirsdottir OG, Ramel A, Gislason T, Gunnarsdottir I. Oral nutrition supplements and between-meal snacks for nutrition therapy in patients with COPD identified as at nutritional risk: a randomised feasibility trial. BMJ Open Respir Res 2019;6:e000349.
- 51. Nichols BL, Alvarado J, Hazlewood CF, Viteri F. Magnesium supplementation in protein-calorie malnutrition. Am J Clin Nutr 1978;31:176-188.
- 52. Fein P, Suda V, Borawsky C, Kapupara H, Butikis A, Matza B, Chattopadhyay J, Avra MM. Relationship of serum magnesium to body composition and inflammation in peritoneal dialysis patients. Adv Perit Dial 2010;26:112-115.
 , Mohammad Hassan Eftekhari2
, Mohammad Hassan Eftekhari2 , Zohreh Mazloom2
, Zohreh Mazloom2 , Masoom Masoompour3
, Masoom Masoompour3 , Mohammad Fararooei4
, Mohammad Fararooei4 , Morteza Zare5
, Morteza Zare5 , Najmeh Hejazi2
, Najmeh Hejazi2