ABSTRACT
Hemodialysis (HD) patients can experience appetite alterations that affect meals and nutritional status. Few qualitative studies have assessed the chronic impact of HD on the everyday diet. This study aimed to characterise comprehensively the experiences of HD patients adapting to appetite alteration. Semi-structured, face-to-face interviews were conducted in a unit of a tertiary hospital to understand patient experiences with appetite alteration. An interview guide was used to consider adaptive processes developed after reviewing the literature and based on the researchers’ clinical experiences. A single researcher conducted all interviews to maintain consistency in data collection. The interview content was analysed using Nvivo 11 based on grounded theory and constant comparison analysis. As a results, the mean age and HD vintage of 14 participants were 60 and 5.8 years, respectively. We developed a self-care model based on HD patient experiences with appetite alteration based on axial and selective coding. Differences in urea sensitivity, taste alteration, and social support could be explained by timing of transitions, life events, and responses to stress. Self-care processes are adapted through the processes of “self-registration” and “self-reconstruction,” starting with “disruption.” At the stage of adjustment, 4 self-management types were derived based on pattern of self-care: self-initiator, follower, realist, and pessimist. The results of this study provide unique qualitative insight into the lived experiences of HD patients experiencing appetite alteration and their self-care processes. By recognising dietary challenges, health teams can better support HD patients in the transition from dietary education to self-care.
-
Keywords: Appetite; Grounded theory; Hemodialysis; Self-care; Social support; Taste
INTRODUCTION
Among hemodialysis (HD) patients, as many as 50% experience protein-energy wasting [
1,
2,
3]. Many previous studies have addressed biochemical mechanisms for appetite among chronic kidney disease (CKD) patients [
4,
5,
6]. However, appetite changes reported by patients vary and are affected by numerous factors, and studies of appetite are subjective and complex [
7]. Xerostomia, which is a medical term for dry mouth, is observed frequently in HD patients, with prevalence ranging from 28%–67% depending on the instruments used for assessment [
8]. Xerostomia is related to poor appetite [
9] and influences alterations in taste perception and nutritional status in HD patients [
10]. Recently, studies have examined the pathophysiology and management of taste alteration in CKD [
11], but the literature presents only a limited understanding of changes in appetite and taste among HD patients. Although clinical tests of perception are available [
12], there is a lack of guidance for prescribing practices to address taste changes in CKD.
Most studies have assessed appetite through cross-sectional research using appetite questionnaires [
12]. There is limited research examining the experience and self-management of appetite alteration among HD patients and a lack of established theory to explain such alteration. Therefore, the aims of this study were to describe the perspectives and experiences of HD patients regarding appetite alteration and to understand their self-management for dietary adherence to identify strategies for improving nutritional care.
MATERIALS AND METHODS
Prior to this study, an examination of a nutrition screening tool for HD patients [
13] and a qualitative study of problems in nutritional care [
14] were conducted in the HD unit of University Hospital. To explore patient perspectives regarding appetite and taste, we conducted in-depth, face-to-face, individual interviews with the same HD patients included in these previous studies. All patients were over 18 years of age and had been undergoing maintenance HD therapy for more than 3 months. HD patients were selected to participate using purposive sampling. From 80 participants who received nutritional care services, we selected patients for inclusion in this study until we reached the level of data saturation. The final number of patients included in our sample was 14. All participants provided written informed consent prior to the interview. The study protocols were approved by the institutional review board of Ajou University Medical Center (AJIRB-MED-SUR-17-138).
The interview guidelines consisted of 4 parts and included semi-structured interview questions (
Table 1) based on previous studies and professional experience in nutrition care. Interviews were conducted from July to September 2017. A trained dietitian carried out all interviews in the HD room. After initial interviews (30–60 minutes), during which food cards were used to help patients visualize and explain their food preferences, the dietitian asked patients to take mobile phone pictures of their usual meals and food intake. The second interview lasted from 15–30 minutes and discussed pictures of food received from patients. All interviews were audio-recorded digitally and transcribed verbatim.
Table 1 Examples of questions about dietary experience in in-depth interviews with hemodialysis patients
Table 1
|
Areas |
Subjects |
Questions |
|
Experience in dietary control |
Dietary education from medical staff |
How did you receive your dietary education from the medical staff (MD, RD, RN)? |
|
Information from other sources |
How do you obtain dietary information outside of the hospital (social gatherings, internet websites)? |
|
Difficulty in diet compliance |
Please tell me about your difficulty restricting food, including meals and fluids. |
|
Reasons for poor diet control |
Why do you think that you cannot manage your diet well? |
|
Appetite alteration |
Appetite changes over dialysis duration |
Please describe how your appetite has changed since you started dialysis. |
|
Appetite cycle depending on dialysis schedule |
Was your appetite different before and after dialysis? What kinds of foods do you eat right after dialysis? |
|
Appetizing food |
What kinds of foods do you usually eat to increase your appetite? (subjective feeling or strength) |
|
Other symptoms |
Do you have symptoms other than anorexia? |
|
Appetite-boosting medications |
Do you have any experience using appetite stimulants? If so, was the medication effective? |
|
Taste alteration |
Sensory alteration |
Do you have any specific food that you craved before or after dialysis? |
|
Please describe in more detail how you adjusted for changes in taste and smell (duration, pattern). |
|
Metal taste |
Have you experienced metallic taste when using a metal spoon? Have you ever used other types of utensils to avoid the metallic taste? |
|
Dry mouth |
Have you ever had trouble eating dry food or a certain food? |
|
Empirical solutions |
How did you resolve this difficulty? |
|
Resources for success |
Social support |
Who can help you maintain your eating and drinking habits? |
|
Clinical support |
What can be offered by medical staff or a peer group? |
|
Suggestions for healthy eating |
What more do you want to know about eating well? |
Using the principles of grounded theory, the transcripts were interviewer coded using QSR NVivo 11 software (QSR International, Doncaster, Australia), and inductively identified concepts related to appetite alteration were identified. Field notes and memos provided contextual information during the constant comparison analysis process through which we drew theoretical insights. This was followed by an independent analysis of the transcripts by 2 of the members of the research team who had considerable expertise in clinical nutrition. They discussed coding choices and performed critical comparisons of themes until a consensus was reached.
Two strategies were used to determine validity in this study. The first is member checking because the experiences of HD patients are the most important items contained in the text. During data collection and analysis, the researcher regularly asked questions and confirmed answers on long-term dialysis with HD patients who had a clear understanding of the purpose of the study. The other strategy is triangulation. We included HD patient interviews, pictures of their meals, and peer review with medical staff. A code-recode strategy was used to support dependencies [
15].
RESULTS
Among the 15 participants who were initially contacted, 14 (93%) participated, after one refused. The mean age and HD vintage of the participants were 60 (40–80) and 5.8 (0.5–13) years, as shown in
Table 2.
Table 2 Demographic and clinical characteristics of 14 HD patients participating in semi-structured interview data collection on appetite alteration
Table 2
|
Participant |
Sex |
Age (yr) |
Length of HD (yr) |
Duration of dialysis (yr) |
Diabetes/CVD history |
Remark |
|
1 |
F |
45 |
0.5 |
4.5 |
No/No |
Peritoneal dialysis started in 2013 |
|
HD initiated after failure |
|
2 |
M |
57 |
1 |
1 |
No/No |
Kidney transplantation in 2006 |
|
HD initiated after organ rejection |
|
3 |
F |
64 |
3 |
3 |
No/No |
|
|
4 |
F |
70 |
4 |
4 |
No/No |
|
|
5 |
M |
61 |
4 |
4 |
Yes/Yes |
|
|
6 |
F |
61 |
5 |
5 |
No/No |
Kidney transplantation in 2002 |
|
After rejected due to chemotherapy, HD |
|
7 |
F |
40 |
5 |
5 |
No/No |
|
|
8 |
M |
63 |
5 |
5 |
No/No |
|
|
9 |
M |
52 |
5 |
5 |
No/No |
|
|
10 |
M |
53 |
6 |
6 |
No/Yes |
|
|
11 |
F |
58 |
8 |
8 |
No/No |
|
|
12 |
F |
66 |
10 |
10 |
Yes/No |
|
|
13 |
F |
70 |
11 |
11 |
No/No |
|
|
14 |
F |
80 |
13 |
13 |
No/No |
|
In the open coding process, properties and dimensions of 72 concepts, 31 sub-categories, and 11 categories were derived, as shown in
Table 3. The paradigm model was identified by axial coding, as shown in
Table 3, and selective coding, as shown in
Figure 1.
Table 3 Concepts, categories, sub-categories, and axial open coding from in-depth interviews of long-term hemodialysis patients’ chronic appetite alteration experience
Table 3
|
Axial coding |
Category |
Sub-category |
Concept |
|
Causal condition |
Minimal kidney function |
Diagnosis of end-stage renal disease |
• Kidney function slowly declined due to other diseases |
|
• Sudden discovery of end-stage renal disease in an emergency room |
|
No enduring uremic symptoms |
• Difficulties eating due to persistent loss of appetite |
|
• Abnormal activity because of cardiovascular events, shortness of breath, or oedema |
|
Phenomena |
Fluctuating appetite |
Inconsistent appetite |
• Unpredictable appetite change |
|
• Persistent poor appetite due to slow recovery |
|
• Difficulty in controlling meals due to a good appetite |
|
Appetite suppression by treatment |
• Loss of appetite from limited food choices based on dietary restrictions |
|
• Poor appetite because of constipation caused by medications |
|
• Skipping meals because of interdialytic weight gain |
|
Context condition |
Sensitivity of urea removal |
Decreased appetite as dialysis duration increases |
• The dramatic experience of uremic removal from the initial dialysis |
|
• Reduced effect on appetite after recent dialysis compared to early dialysis |
|
Appetite cycle according to dialysis schedule |
• Skipping meals because of loss of appetite just before dialysis |
|
• Hunger due to a good appetite immediately after dialysis |
|
• Snacking instead of having a meal shortly after dialysis, resulting in weakness |
|
Taste alteration |
Sensitive taste/Smell |
• Changes in taste after dialysis |
|
• Avoiding eating out because of smells |
|
• Sensitive to the metallic taste of red meat |
|
Discomfort due to dry mouth |
• Indigestion because of lack of saliva |
|
• Prefer moist food over lean meat for better swallowing |
|
Picky eater |
• Returning to familiar foods |
|
• Noncompliance to strict hospital meals |
|
Intervening conditions |
Social support |
Emotional support |
• Psychological support for anorexia |
|
• Skipping meals because of living alone or weakness |
|
Belonging support |
• Enjoying a meal with friends |
|
• Participation in religious activities |
|
Tangible support |
• Providing meals for patients |
|
• Getting family involved in housework |
|
Information support |
• Providing information for recovery |
|
• Comprehensible prescription |
|
Action and interactional strategies |
Self-recognition |
Eager to learn |
• Attention to test results and drug prescriptions |
|
• Specific questions about meal plan |
|
• Interest in new information and requests individual counselling |
|
Dietary compliance |
• Understanding the relationship between diet control and test results |
|
• Focusing on adjusting the amount of food rather than dietary restrictions |
|
• Regular and balanced meals |
|
Healthy habit |
• Habitual exercise for appetite and muscle strength |
|
• Participation in social gatherings |
|
• Avoiding processed foods |
|
Self-reconstruction |
Knowledge |
• Tips for favourite food intake |
|
• Effect of nourishing foods |
|
• Effect of appetite stimulants |
|
Self-efficacy |
• Continuous adjustments |
|
• Maintaining normal taste and appetite |
|
• Request for help and proper refusal |
|
Self-monitoring |
• Consistency in weight gain around dialysis |
|
• Appropriate calories and protein intake |
|
• Check the test results while controlling medications |
|
Consequences |
Self-initiator |
Meal similar to ordinary people |
• Not fussy about food |
|
• Maintaining fitness and a good appetite |
|
Being self-empowered |
• Maintaining good test results with good habits |
|
• Independence from medical staff |
|
Partnership in care |
• Describing current symptoms to medical staff |
|
• Understanding the medical explanation |
|
Follower |
Strict diet compliance |
• Gratitude for life extension because of dialysis |
|
• Diet control is as important as taste |
|
Need adaptation process |
• Self assessment after trying new foods |
|
• Adapting to dialysis situation |
|
Having trust in medical staff |
• Easy access to practical education |
|
• Verifying inconsistent information with healthcare providers |
|
Realist |
Uncontrolled eating |
• Unplanned diet and abnormal test results |
|
• Weight gain because of cravings |
|
Optimistic personality |
• Considering themselves as young and having a large appetite |
|
• Does not refuse temptation of alcohol and uncontrolled overeating from lack of will |
|
Negative evaluation from medical staff |
• No response to test results and non-adherence to medications |
|
• Only caring about weight on dialysis day |
|
Pessimist |
Disinterest in meals due to poor appetite |
• Irregular meals or snacks |
|
• Persistent poor appetite because of taste alteration and dry mouth |
|
Exhausted by long dialysis |
• Difficulty in daily activities and meal preparation |
|
• Low energy and delayed recovery because of accompanying diseases |
|
Dependence due to loneliness |
• Nurses treating patients like a family |
|
• Living alone but supported by independent children |
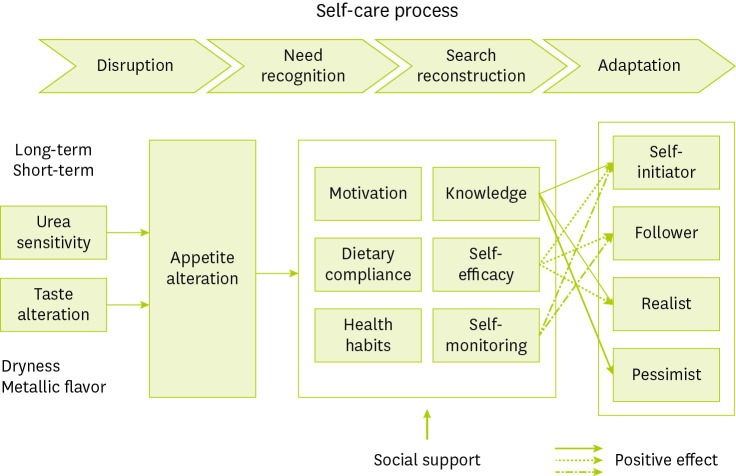
Figure 1 An appetite alteration model for hemodialysis patients based on a combination paradigm (selective coding) with hypothetical formalization from in-depth interviews. Time-based adaptation period was divided into 4 stages of disruption, need recognition, search reconstruction, and adaptation. During “disruption,” patients experience a fluctuating appetite with the sensitivity of urea removal and taste alteration. The second stage, “need recognition,” refers to a period where the patients obtained motivation, dietary compliance, and health habits. The third stage, “search reconstruction,” is a period when the participants obtained knowledge, self-efficacy, and self-monitoring skills. Finally, “adaptation” is related to appetite alteration and is categorised into 4 types based on positive affects (marked with arrows) and self-management categories. The 4 self-management types are derived from the attributes and dimensions of each category. The self-initiator type had positive dimensions for knowledge, self-efficacy, and self-monitoring. The follower type had positive dimensions for self-efficacy and self-monitoring because of a relatively short dialysis duration. The realist type had positive dimensions for knowledge and self-efficacy because of lack of self-monitoring. The pessimist type had positive dimension for knowledge because they were knowledgeable but uncooperative in dialysis, resulting in low self-efficacy and lack of self-monitoring.
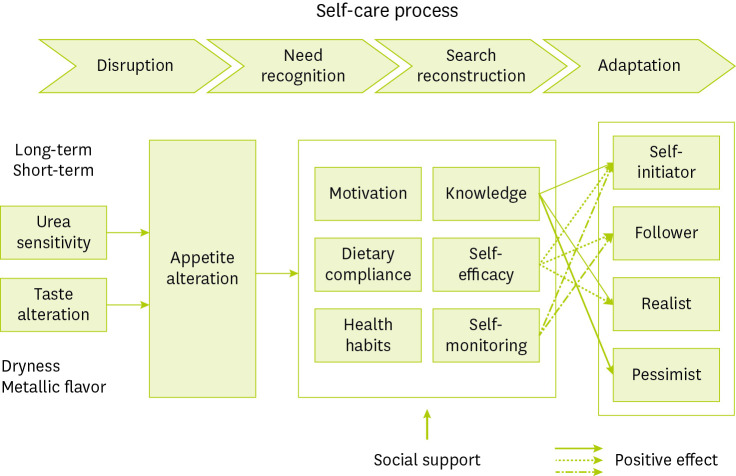
Minimal kidney function
Minimal kidney function was derived as a causal condition. We recognised that kidney function slowly declined and acknowledged the necessity of terminal stage therapy. Two participants experienced recurrent symptoms of end-stage kidney disease (ESKD) after rejection of kidney transplants.
As ESKD progressed for more than 1 year, maintaining a usual eating pattern became challenging for patients because of uremia symptoms compared to the early CKD stages. Some participants with a short duration of kidney failure had no difficulty eating because they had not experienced poor appetite but had trouble with their daily activities because of edema or shortness of breath.
Participant 8: I experienced a loss of appetite after I was diagnosed with CKD, but I did not have any difficulties in eating at the beginning of dialysis about 5 years ago. ……… While I was working, I could not even eat because I felt sick whenever I smelled food.
Fluctuating appetite
Fluctuating appetite was derived as a core phenomenon. Participants reported poor appetite because of flu or seasonal changes and experienced loss of appetite even when they worked harder than usual. When their energy levels were low with no clear cause, they reported a sudden loss of appetite. To address these problems, one participant often ate small amounts of fruits that were supposed to be restricted and used nutritional supplements or an appetite stimulant. Some participants ate nourishing foods or meats to replenish energy. Just before the day of dialysis, patients sometimes skipped meals because of poor appetite but felt hungry and then overate on non-dialysis days. A few elderly participants who did not have an appetite even after dialysis ate the minimum amount of food for survival on the day of dialysis. Unlike the elderly, middle-aged participants had good appetites and difficulty in refraining from eating. Some participants always had a good appetite and were willing to eat certain foods that generally were restricted such as fruits or coffee.
Participant 12: My appetite fluctuates without any specific reasons. Sometimes I eat a lot of food because I have a good appetite after dialysis. On the other hand, I sometimes cannot even eat anything due to exhaustion after dialysis. ……… I feel hungry but do not eat anything in the morning on dialysis day to control my weight.
While regular dialysis improved appetite, strict diet restrictions led to poor appetite and a long-term risk of malnutrition. Medication side effects of constipation and indigestion affected participants’ ability to eat regularly. Interdialytic weight gain led to irregular eating patterns that often caused patients to skip meals or overeat after dialysis.
Participant 6: It seems like dialysis patients tend to lose muscle mass. I think it is because patients are not able to eat well for a long time when they realize there are many dietary restrictions even though they had a good appetite at the beginning of dialysis.
Sensitivity of urea removal and taste alteration
Sensitivity of urea removal and taste alteration were derived as context conditions. Depending on the participant, an adjustment period seemed to be necessary at the beginning of dialysis, but most participants experienced rapid appetite increase at the beginning of dialysis. Over the period of dialysis, appetite declined. Elderly participants with longer dialysis complained about lack of energy more than lack of appetite.
On the day of dialysis, poor appetite was noted right before dialysis. However, appetite increased after dialysis, and most participants were able to eat regardless of taste of the food. Some participants who could not tolerate hunger until they returned home ate ready-to-eat lunches or snacks after the procedure. Other participants were lethargic after dialysis and so skipped meals or replaced meals with snacks.
Participant 8: On dialysis day, I do not have any appetite in the morning. However, in the evening after dialysis, I have a good appetite and I feel hungry. . . . I eat a small breakfast, and I feel hungry after dialysis.
Before dialysis, fish, meat, and oily food were eaten well. However, the longer was the dialysis treatment, the larger was the change in participant food preferences. As the number of foods that participants preferred decreased, the number of unbalanced meals increased. Because of the metallic flavour of red meat, spicy marinated meats were preferred over plain meat. Participants with a good appetite were less sensitive to taste and smell.
Participant 6: I especially disliked meats after kidney failure. I did not like the smell of meat, and I felt nauseated after eating them. I could not eat pork, fatty food, or greasy food anymore. I could not even eat any meats that were not marinated. When the meat was marinated with spicy sauce, I was able to eat because of spicy seasonings.
Because of a lack of saliva, both indigestion and difficulty in swallowing were noted. Participants these side effects preferred moist foods over lean meats. Participants who ate controlled meals were thirsty and consumed more water than high-calorie foods. As a result, longer dialysis treatment made it difficult for participants to resolve their thirst. Fatty meats or recipe changes to moister food were preferred over boiled lean meats.
Participant 13: I have a fissured tongue. I do not have enough saliva. I know the smell of food and the taste, but I just swallow even though I do not recognise the real taste. I do not think I can digest well. I think it is because I have a dry mouth, and my tongue is cracked. I can eat soft cake because it is soft although it smells like eggs. Choco-pie tastes good, but I need to sip water while eating the pie. I cannot eat without water.
Participants who had to control their diet preferred comfortable and familiar foods that they had enjoyed previously. Participants rejected strict therapeutic diets, such as hospital meals, and wanted to choose their own foods.
Participant 9: I have not eaten anything raw since I was young, which is the reason why I do not eat raw fish or beef. I like any food made of tofu. I have liked boiled-down foods in sauces as well as pan-fried food since I was young.
Social support
Social support was derived as an intervening condition. In emotional support, participants’ children or nurses encouraged them to eat well. Participants had trouble preparing meals but tried to eat more because of such encouragement, despite the burden of cooking.
Participant 13: I have been living alone for 11 years. My independent daughter and son keep telling me eat more because they think I do not eat enough. I avoid eating salty foods so they do not worry about that. I just try to eat more because I was told to by my nurse. My problem is poor appetite.
Participants were grateful when families chose meals in consideration of participant enjoyment. Participants felt joy while eating delicious food with close friends.
Participant 4: I did follow a therapeutic diet at first; however, I go out to eat and search for nice restaurants nowadays. I do not restrict my diet when my friends treat me to some food outside my home. My financial situation is not so great; therefore, I cannot treat myself to high-quality meats. Thus, I eat some meats when my friends buy them for me.
As intangible support, participants’ spouses sometimes prepared meals or bought food. If the participant was sick, their family did their housework.
Participant 6
:
During dialysis, there is no problem when I feel good. However, there are some days that I am lethargic and have low energy quite often. When I feel muscle aches such as getting a cold, my energy goes down. I cannot even eat because I have a poor appetite and I cannot even walk because I have no energy. When I clean the house, my energy drops completely. I cannot do anything by myself. I need my family who can help me.
Regarding information support, participants received basic education at the start of dialysis but needed a guide to answer questions or address difficulties while maintaining dialysis. Participants expressed their gratitude to the medical staff who evaluated their overall health and provided personalised care.
Participant 10: My wife is interested in how taste changes after dialysis. She knows a lot because she researches dietary information online. She tells me the information that she found, and she follows it well.
Self-recognition and self-reconstruction
Self-recognition and self-reconstruction were derived as action and interactional strategies. The subcategory of eager-to-learn participants was interested in the results of regular check-ups and prescription drugs. Participants who had dialysis asked specific questions of the dietitians or searched for the information they needed in other ways.
Participant 1: After I came here, I requested to meet a dietitian. The dietitian informed me about general information for dialysis patients, which was different than what I expected. I can search for general information online because I can find it on my own, because I am interested as well. I also use a mobile application that shows the amount of potassium and phosphorous based on each ingredient.
In the subcategory of dietary compliance, participants were unable to eat their favourite foods; however, those who had undergone dialysis for long periods of time controlled the amount rather than types of food. Participants with normal blood results reported intake of restricted foods. Some participants were well adapted to regular meal patterns, but others said that it became a habit to base meal pattern on dialysis schedule.
Participant 4: I try not to skip meals. I eat meals regularly, but I do not eat snacks. I want to drink coffee or eat some fruits, but I try not to. I try to eat meals 3 times a day, although my portion size is small.
In the sub-category of healthy habits, participants tried to avoid eating out, processed foods, raw fish, and functional foods. Participants exercised before meals to stimulate their appetite and exercised regularly to maintain muscle strength. Participants said they maintained a good appetite because of their busy social life.
Participant 1: I did not eat Korean-style seasoned chicken, which has strong flavor with spicy sauces. Whenever I eat strongly seasoned food from outside my home, I do not feel well. Thus, I try to avoid eating these foods. I think food additives affect my health.
The subcategory of knowledge participants acknowledged how to safely eat their favorite foods while controlling their amounts and frequencies. Participants reported experiences of recovery by eating nourishing food such as grilled meat, chicken soup, and pork stew. Participants also requested their medical doctors provide them appetite stimulants or oral nutritional supplements when they were experiencing weakness.
Participant 2: I eat beef a lot. I eat beef 3 to 4 times a week. When I buy 500 g of beef, I think I eat about 120 g because I buy it for 4 people. Mostly, grilled beef steak stimulates my appetite.
The sub-category of self-efficacy participants controlled their diets by monitoring interdialytic weight gain and blood testing. When participants felt exhausted, they stopped doing routine household chores to rest and asked their families for help. One participant rejected her daughter's requests to take care of her grandson when she experienced weakness.
Participant 12: Now I know how much I am supposed to eat. I realised how I should do; therefore, I feel better when I eat food. I currently control my diet even though I was told not to eat much. I can manage portion size by myself because I know the safe amount of food that I can eat.
The subcategory of self-monitoring participants measured their weight frequently and maintained a constant weight by adjusting their diet. Participants did not skip meals to maintain fitness. Participants planned to regularly consume a certain amount of protein during meals. With controlling medications, participants monitored the effects and side effects through blood tests.
Participant 8: I was recommended that I should stay away from eating out and restrict my diet. I know which foods are not good for me and I eat selectively. I just learned from my experience and I think I know what I should eat without restricting my diet. It does not bother me when it comes to food. I weigh myself a few times every day. I control my portions based on my weight.
Self-initiator, follower, realist, and pessimist categories
Self-initiator, follower, realist, and pessimist categories were derived as consequences.
Participants in the self-initiator category possessed internalising knowledge, high self-efficacy, and overall self-monitoring. These participants developed self-confidence and empowerment by becoming their own therapists and relying less on the medical staff. Participants desired normality in their life roles and functions (Participants 6, 8, 12).
Participant 6: I had a lot of questions at first. I take a medication to decrease my level of phosphorus. Therefore, I eat without any concerns. I have never missed taking the medication. I try to be careful because I will need to take more medications when my test level goes up. When I pay attention, my level goes down.
Participants in the follower category had little knowledge, high self-efficiency, and overall good self-monitoring. Followers were grateful for increases in appetite resulting from dialysis and tried to maintain strict dietary restrictions. During adaptation to dialysis, these participants tried to adjust to the environment of the dialysis room and tried new foods, but they went through a verification process as stated below (Participants 1, 2, 3, 4, 11).
Participant 1: I realised I have never thought about appetite because there was no one who mentioned the importance of appetite. I have been thinking that I cannot eat what I want for a long time. It was new to me learning food preparation techniques such as soaking food in water beforehand. If I had known earlier, I would have eaten more vegetables and potatoes and I would not have lost 3 kg.
The realists had knowledge, high self-efficiency, and partial self-monitoring. Realists had knowledge of proper nutrition and were performing some self-management despite negative evaluations from medical staff. However, they thought of themselves as young and healthy and were optimistic about the future. These participants showed high self-efficacy despite frequent mistakes (Participants 5, 7, 9, 10).
Participant 5: I thought I could lose weight easily because I used to work out regularly. It has been a while because I want to eat a lot and I am still young. I do not think I am good at managing my weight. I lost more weight than my dry weight, which resulted in lowering my energy. I recently restrained myself from eating potatoes because of potassium. Although I know that I should soak ingredients in water for 2 hours in order to reduce potassium before cooking, it does not work the same way that they recommended. I just cook based on my hunger and appetite each day.
Participants in the pessimist category had experienced long periods of dialysis, were older, and lived alone. Pessimists felt powerless despite their knowledge, exhibited weak self-efficiency, and maintained partial self-monitoring. They complained of discomfort during food intake while experiencing poor appetite for a long time. Pessimists took part in self-management and ate simple meals with an unbalanced diet because of low energy. They relied on medications such as appetite stimulants and were ambivalent towards treatment. Such participants were comforted by compassionate nurses because of their loneliness (Participants 13, 14).
Participant 13: I think other patients have no problem with eating. I eat when I need to eat, but I eat just enough to maintain my life. I think what I eat is not good enough. When I was admitted to hospital due to pneumonia, I had a poor appetite so I had to take an appetite stimulant. I do not have any food that I want and I eat meals with one or 2 side dishes, which I think is not good enough. I just eat because my nurse told me to.
Selective coding was used to identify the self-care process related to appetite as the core category. These properties were sensitivity of urea removal, degree of taste alteration, types of functional supports, levels of knowledge and self-efficacy, and degree of self-monitoring. During search reconstruction, the levels of knowledge and self-efficacy ranged from low to high, and the degree of self-monitoring ranged from partial to overall. The self-care process begins with disruption, progresses to needs recognition and search reconstruction, and eventually reaches adaptation through action and interactional strategies. As a result, 4 types of self-management were classified into 4 groups, as shown in
Figure 1.
DISCUSSION
In this study, we gained insight into the experiences of patients living with appetite alteration after HD treatment. Our findings suggest that HD patients who experience appetite cycles associated with sensitivity to urea and taste alteration can be divided into 4 types of self-management through the self-care process and social support networks [
16]. The responses of our HD patients were similar to those in a previous review of taste studies [
11]. However, in clinical settings, progression of disease, individual sensitivity in terms of taste and urea removal, and types of individual social support need to be recognised and understood based on the trajectories of personalized appetite through patient self-care processes. Our results show that taste and appetite change as the disease progresses over the long-term as well as in association with the dialysis schedule.
In this study, knowledge was not merely defined as basic information such as that of restricted food lists [
17], but was based on patient experiences [
18]. Therefore, experience and knowledge were important in self-care processes. Self-efficacy plays a significant role because, the longer is the dialysis period, the lower is patient compliance. Self-monitoring, which is the process of self-checking, interdialytic weight gain, and abnormal blood results, frequently is performed by self-initiator and follower types. Behavioural study has shown that self-monitoring influences dietary adherence [
19] and has led to recent development of a self-monitoring and educational dietary app [
20]. In addition, social and family support promote dietary compliance in HD patients [
21]. Our findings are congruent with those of a systematic review [
22] showing that individuals usually differentiate between tangible sources of support.
The main limitation of this study is its small sample of individuals treated in a single tertiary HD unit. Our observations might not be generalisable to all HD patients. Despite the small number of patients surveyed, the results of this study provide new insights into practice in the field of clinical nutrition as a basis for understanding HD patient conditions and attitudes towards disease process [
23].
More research is needed on the patient experience, including attentive diet coordination based on a mechanistic understanding of pathology among individuals. A qualitative component helped to identify strategies to prevent patients from progressing to the pessimistic stage [
24]. Clinical core performance can be applied to the following areas. Nutrition education should be approached through an individualised condition based on appetite, taste, and symptoms as well as social support. Dietary recommendations to manage taste change have aimed to reduce the metallic flavor in lean red meat using seasoning and enhanced moisture conditions. In addition to nutritional factors, dietitians should consider self-management types based on patterns of self-care to help improve patient’s nutritional status.
NOTES
-
Conflict of Interest: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Hwang W, Oh J, Park I, Cho MS.
Formal analysis: Hwang W, Lee JH, Nam J.
Writing - original draft: Hwang W, Nam J.
Writing - review & editing: Hwang W, Lee JH, Nam J, Oh J, Pakr I, Cho MS.
REFERENCES
- 1. ESRD Registry Committee of Korean Society of Nephrology (KSN). Current renal replacement therapy in Korea [Internet]. 2021. cited 2022 Oct 25. Available from https://ksn.or.kr/bbs/index.php?code=report
- 2. Lim HS, Kim HS, Kim JK, Park M, Choi SJ. Nutritional status and dietary management according to hemodialysis duration. Clin Nutr Res 2019;8:28-35.
- 3. Wright M, Southcott E, MacLaughlin H, Wineberg S. Clinical practice guideline on undernutrition in chronic kidney disease. BMC Nephrol 2019;20:370.
- 4. Carrero JJ, Stenvinkel P. Chapter 38. Anorexia and appetite stimulants in chronic kidney disease. In Kopple JD, Massry SG, Kalantar-Zadeh K, eds, ddNutritional management of renal disease. 3rd ed. Amsterdam: Elsevier; 2013, pp 645-659.
- 5. Mckeaveney C, Noble H, de Barbieri I, Strini V, Maxwell AP, Reid J. Awareness, understanding and treatment practices when managing cachexia in end-stage kidney disease. J Ren Care 2020;46:35-44.
- 6. Grove BE, Schougaard LM, Hjollund NH, Ivarsen P. Self-rated health, quality of life and appetite as predictors of initiation of dialysis and mortality in patients with chronic kidney disease stages 4-5: a prospective cohort study. BMC Res Notes 2018;11:371.
- 7. Cupisti A, Brunori G, Di Iorio BR, D’Alessandro C, Pasticci F, Cosola C, Bellizzi V, Bolasco P, Capitanini A, Fantuzzi AL, Gennari A, Piccoli GB, Quintaliani G, Salomone M, Sandrini M, Santoro D, Babini P, Fiaccadori E, Gambaro G, Garibotto G, Gregorini M, Mandreoli M, Minutolo R, Cancarini G, Conte G, Locatelli F, Gesualdo L. Nutritional treatment of advanced CKD: twenty consensus statements. J Nephrol 2018;31:457-473.
- 8. Bossola M. Xerostomia in patients on chronic hemodialysis: an update. Semin Dial 2019;32:467-474.
- 9. Bossola M, Di Stasio E, Giungi S, Vulpio C, Papa V, Rosa F, Tortorelli A, Tazza L. Xerostomia is associated with old age and poor appetite in patients on chronic hemodialysis. J Ren Nutr 2013;23:432-437.
- 10. Lynch KE, Lynch R, Curhan GC, Brunelli SM. Altered taste perception and nutritional status among hemodialysis patients. J Ren Nutr 2013;23:288-295.e1.
- 11. Brennan F, Stevenson J, Brown M. The pathophysiology and management of taste changes in chronic kidney disease: a review. J Ren Nutr 2020;30:368-379.
- 12. Márquez-Herrera RM, Núñez-Murillo GK, Ruíz-Gurrola CG, Gómez-García EF, Orozco-González CN, Cortes-Sanabria L, Cueto-Manzano AM, Rojas-Campos E. Clinical taste perception test for patients with end-stage kidney disease on dialysis. J Ren Nutr 2020;30:79-84.
- 13. Hwang W, Cho MS, Oh JE, Lee JH, Jeong JC, Shin GT, Kim H, Park I. Comparison of creatinine index and geriatric nutritional risk index for nutritional evaluation of patients with hemodialysis. Hemodial Int 2018;22:507-514.
- 14. Song H, Hwang W, Kim WJ. Experiences of nurses working at hemodialysis centerson education of self care diet therapy for hemodialysis patients. Health Nurs 2019;31:53-62.
- 15. Corbin J, Strauss A. Basics of qualitative research: techniques and procedures for developing grounded theory. New York: Sage publications; 2014.
- 16. Christensen AJ. Patient-by-treatment context interaction in chronic disease: a conceptual framework for the study of patient adherence. Psychosom Med 2000;62:435-443.
- 17. Gibson EL, Held I, Khawnekar D, Rutherford P. Differences in knowledge, stress, sensation seeking, and locus of control linked to dietary adherence in hemodialysis patients. Front Psychol 2016;7:1864.
- 18. García Montes JM, Sánchez Elena MJ, Valverde Romera M. The influence of coping and personality styles on satisfaction with life in patients with chronic kidney disease. Psychol Belg 2020;60:73-85.
- 19. Howren MB, Kellerman QD, Hillis SL, Cvengros J, Lawton W, Christensen AJ. Effect of a behavioral self-regulation intervention on patient adherence to fluid-intake restrictions in hemodialysis: a randomized controlled trial. Ann Behav Med 2016;50:167-176.
- 20. Fakih El Khoury C, Karavetian M, Halfens RJ, Crutzen R, El Chaar D, Schols JM. Dietary application for the management of patients with hemodialysis: a formative development study. Healthc Inform Res 2019;25:262-273.
- 21. Oquendo LG, Asencio JM, de Las Nieves CB. Contributing factors for therapeutic diet adherence in patients receiving haemodialysis treatment: an integrative review. J Clin Nurs 2017;26:3893-3905.
- 22. Sousa H, Ribeiro O, Paúl C, Costa E, Miranda V, Ribeiro F, Figueiredo D. Social support and treatment adherence in patients with end-stage renal disease: a systematic review. Semin Dial 2019;32:562-574.
- 23. Stevenson J, Tong A, Gutman T, Campbell KL, Craig JC, Brown MA, Lee VW. Experiences and perspectives of dietary management among patients on hemodialysis: an interview study. J Ren Nutr 2018;28:411-421.
- 24. Raj R, Brown B, Ahuja K, Frandsen M, Jose M. Enabling good outcomes in older adults on dialysis: a qualitative study. BMC Nephrol 2020;21:28.