ABSTRACT
Non-alcoholic fatty liver disease (NAFLD) is a significant public health problem globally and the most notable chronic liver disease in Asian countries. Various dietary supplements have been assessed as potential methods to alleviate the metabolic damages related to NAFLD, but the results of these works have been equivocal. This study aimed to evaluate the effects of probiotic yogurt fortified with vitamin D (Pro-YFD) on glycemic and anthropometric indices in patients with NAFLD. One hundred and four NAFLD patients of both sexes were randomly allocated to 2 groups: group A (Pro-YFD) and group B (unfortified yogurt). The intervention period was 3 months. Fasting blood samples were obtained for measuring fasting blood sugar (FBS) and insulin level. Food intake was measured using a validated food frequency questionnaire. Body composition was estimated by bio-impedance. Eighty-eight patients completed the study. The mean serum level of 25(OH)D3 was elevated significantly (p < 0.001), while insulin level decreased significantly (p < 0.003) in group A at the end of the study. FBS levels showed no significant differences between the groups at the end of the trial. Also, there were no significant changes in diet caloric intake, physical activity, or anthropometric indices in the 2 groups during the interventions. Pro-YFD in the diets of patients with NAFLD may attenuate insulin resistance and improve serum level of 25(OH)D3.
-
Keywords: Glycemic index; Yogurt; Non-alcoholic fatty liver disease; Vitamin D
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is an inflammatory disease, refers to a range of diseases associated with over-fat accumulation in the liver in people who do not intake alcohol consumption [
1,
2,
3]. NAFLD has become a public health problem in the world [
4] and is progressively becoming the most significant chronic liver disease not only in Western countries [
5], but also in Asia [
6]. Currently, there are no proven treatments for NAFLD, but lifestyle modifications, especially dietary changes, are essential in the management of these patients [
7,
8]. One of the effects of metabolic disorders are inflammatory outcomes, and some nutrients can be effective in alleviating inflammation due to their metabolic effects. Inflammation not only exacerbates NAFLD but can also lead to adverse systemic effects such as cardiovascular diseases. Modification of inflammation is considered as a new therapeutic target for NAFLD [
9].
Dietary modification at various stages of life is beneficial for maintaining health [
10]. Some nutrients and functional foods, such as probiotics, have anti-inflammatory roles, probiotics are living microorganisms with beneficial effects on the host by improving the microflora of the intestine [
11] and modulate lipid metabolism and inflammatory reactions [
12,
13]. The imbalance of small intestinal bacteria has a severe effect on liver health that occurs in most people with NAFLD [
14,
15]. Dairy products and fermented foods are good sources of probiotics, but today because of using other methods to keep our foods from deterioration, fermentation is not widely used. Nowadays, most foods that people consume contain little or no probiotics due to approaches used in food industrials [
16].
Vitamin D is a fat-soluble vitamin with hormonal function, which is well known for its calcium, phosphorus, and bone metabolism homeostasis [
17]. Vitamin D plays a vital role in decreasing insulin resistance, obesity, pre-diabetes, metabolic syndrome, cardiovascular risk, and oxidative stress [
18]. Therefore, this vitamin can help reduce systemic inflammation by correcting some metabolic disorders [
19]. According to epidemiological studies, Vitamin D deficiency is a common problem among Iranian people [
20,
21,
22,
23]. Besides, evidence has shown that serum concentrations of vitamin D in people with NAFLD are lower than healthy ones [
24]. No studies have so far assessed the impact of vitamin D and probiotics on people with NAFLD. In most studies, nutritional supplements are used to evaluate the effects of nutrients on diseases, but in this study, we used yogurt as a carrier of nutrients to examine its effect on NAFLD. Yogurt is one of the dairy products whose consumption is widespread among Iranian people [
25], so we enriched yogurt with vitamin D and probiotics and evaluated the effect of the vitamin D and probiotic fortified yogurt in patients with NAFLD. This trial was designed to investigate the effect of enriched yogurt with vitamin D and probiotics on serum level of 25(OH)D
3, glycemic control and anthropometric indices in patients with NAFLD.
MATERIALS AND METHODS
Study design
This study was a randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy of vitamin D and probiotic fortified yogurt in subjects with NAFLD in Iran. The sample size was determined based on the effect size of fasting glucose of the previous study [
26] with 90% power and 5% significance. The trial was ethically approved by the Ethics Committee of Kermanshah University of Medical Sciences (grant No. 28809) and registered with the Iranian Clinical Trials Registry (registration No. IRCT20131022015111N3). Written informed consent was obtained from all participants in the beginning of the study.
We designed our study based on the Consolidated Standards of Reporting Trials statement for randomized clinical trials [
27]. Study participants were recruited from Imam Reza Hospital in Kermanshah, western Iran. All participants were diagnosed with NAFLD based on ultrasonography report by internist, the inclusion criteria were NAFLD patients aged between 25–55 years with mild to moderate fatty liver, exclusion criteria included: taking any supplements and medications within 6 weeks [
28] before and during the study, being sensitive to yogurt consumption, having other chronic diseases such as heart, lung, kidney chronic problems or cancers.
A simple random method was used to assign subjects to the intervention or control groups. Using random numbers table, each subject was assigned a number and selected randomly. Ultimately, 104 participants were divided into 2 groups (intervention or control group) by randomization (52 people in each group). Subjects in the intervention group consumed probiotic yogurt fortified with vitamin D (Pro-YFD) (group A), while the control group consumed unfortified yogurt [
29] (group B).
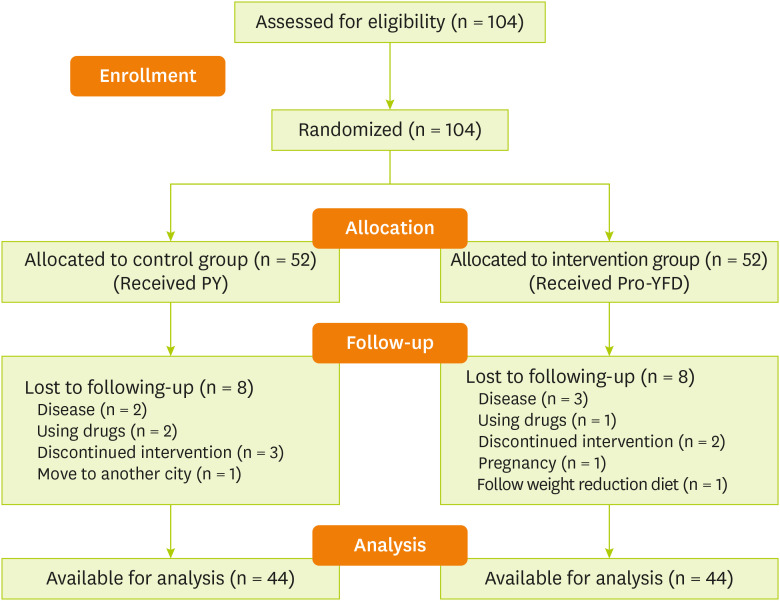
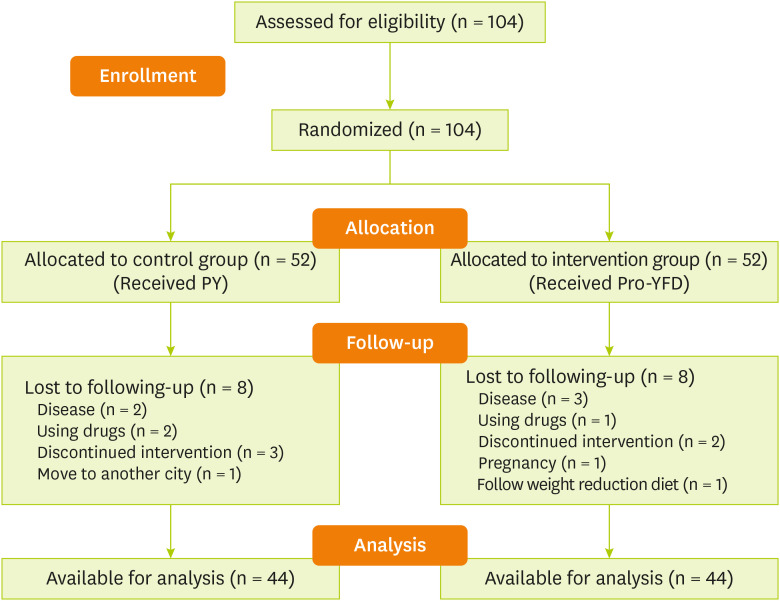
Figure 1 shows the flow chart of the study.
Figure 1
Study flow chart.
PY, probiotic yogurt; Pro-YFD, probiotic yogurt fortified with vitamin D.
Intervention
This parallel-group trial was conducted between 2 groups. Group A consumed 100 g Pro-YFD and group B consumed 100 g probiotic yogurt (PY) every day for 12 weeks. Subjects were fed yogurt every ten days. In each visit, 20 packs of 100 g yogurt were given to participants (10 packs for self-consumption and ten for other family members) to ensure that the subjects would consume the yogurt. Both groups received a form on which they could record their yogurt consumption over ten days. We asked participants to bring back the empty yogurt packs and fill out forms on a future visit. We also asked them to consume yogurt with lunch or dinner. A representative's company coded each yogurt pack that the researcher and the subjects were not aware of the yogurt packs (Pro-YFD or PY).
Process of yogurt fortification
The PY for the control group contains usual primer bacteria, “Streptococcus thermophilus and Lactobacillus bulgaricus and the yogurt for the intervention group,” in addition to the mentioned initiated primers, enriched with Lactobacillus acidophilus La-5 and Bifidobacterium lactis Bb-12 about 4 × 107 cfu22, which was added by Direct Vast Set and fortified with 1,000 international units of vitamin D. The fat content of yogurt was selected to be 1% to create consistency and proper texture. Yogurts in both groups were similar in appearance and taste and were specially prepared for this study by Damdaran Dairy Industries Company.
Subjects were instructed to maintain their regular dietary habits and lifestyle and avoid consuming other probiotic and fermented products or supplements during the study. They also were asked to keep yogurts at refrigerator temperature below 4°C. The stability and amount of vitamin D3 and probiotics in the fortified yogurt were measured on the first and tenth day of each round of the intervention by a private laboratory (PartoBashsash Research Institute, Tehran, Iran) using the high performance liquid chromatography (HPLC) method.
Demographic information
A questionnaire was used to collect demographic information and medical history. Information including age, gender, educational level, occupation, income, daily exposure to sunlight and medical history was gathered through interviews with participants. The questionnaire proved to be valid and reliable in the previous study [
30].
Height was measured with a wall-mounted stadiometer to the nearest 0.1 cm (the shoulders, hips, and heels were in contact with the wall). Weight and body composition of participants were measured by bioelectrical impedance analysis using a body analyzer device (Jawon Medical Plus model Avis 333). Measurements were taken with participants barefoot and wearing light clothing. Body composition measurements included body mass index (BMI), body fat, lean body mass (LBM), and waist to hip ratio (WHR). Waist circumference (WC) was measured using the non-stretched and flexible tape at the iliac crest level with a precision of 0.1 cm at the beginning and end of the intervention. In addition to anthropometric indices, systolic and diastolic blood pressure (SBP and DBP) of participants were measured by a sphygmomanometer.
Biochemical indices
Fasting blood samples (10 mL) were obtained from each participant after an overnight (10–12 hours) fast at the beginning and end of the study. The blood samples were centrifuged, and serum was stored at −40°C until analysis. Fasting blood sugar (FBS) was analyzed by enzymatic method. Fasting insulin concentrations and vitamin D3 were detected by electrochemiluminescence method. In contrast to enzyme-linked immunosorbent assay and HPLC, this method has high sensitivity and specificity. Vitamin D status based on serum levels of 25(OH)D
3 was classified as low (< 20 ng/mL), insufficient (20–29.9 ng/mL) or adequate (greater than 30 ng/mL) [
31].
The homeostatic model assessment for insulin resistance (HOMA-IR) was calculated using the following formula: (HOMA-IR = fasting glucose (mg/dL) × fasting insulin (micro U/mL)/405 [
32].
At the beginning of the study, participants were asked not to make any changes in their diet. Self- administered 3-day food record questionnaire was used to assess the frequency and portion of dietary intake and macronutrients as well as the amount of vitamin D intake through diet (2 days of the week and the weekend) at the first and twelfth week of the study. The amounts of energy and nutrients in foods were measured by nutritionist 4 software using the United States Department of Agriculture Food Composition Table, which was modified for Iranian foods [
33]. A trained dietitian did all data processing and entry.
Physical activity was assessed using the International Physical Activity Questionnaire short form before and after interviewer's intervention. This questionnaire had 7 questions about physical activity associated with work, homework, and leisure during the week. Total metabolic equivalent task per hour per week was calculated based on the instrument existed in the questionnaire. The validity and reliability of the questionnaire had previously been confirmed in Iran [
34].
The quantitative and qualitative variables were expressed as mean ± standard deviation and frequency (number and percent), respectively. The Kolmogorov-Smirnov test was used to assess the normal distribution of quantitative data. The background characteristics and nutrient intakes of participants in the 2 groups were compared using the Mann-Whitney U test or independent sample t-tests. The χ2 test was used for the interpretation of qualitative data. Analysis of covariance was used to identify any differences between the 2 groups after the intervention, adjusting for baseline measurements and confounders. Paired sample t-tests or Wilcoxon compared the changes in biochemical parameters, anthropometric measurements and nutrient intakes between the beginning and end of the intervention. SPSS software version 20 (IBM Corp., Chicago, IL, USA) was used for data analysis. The p values less than 0.05 were considered significant.
RESULTS
This study included 104 individuals; of whom 16 were excluded during the period of intervention due to the indication of gastrointestinal, pneumonia, and chronic kidney diseases (n = 5), discontinuation of intervention (n = 5), using drugs (n = 3), getting pregnant (n = 1), moving to another city (n = 1) and following a weight loss diet (n = 1) (
Figure 1). Ultimately, 88 participants, 41 (46.6%) females and 47 (53.4%) males with an average age of 40 ± 6 years, were enrolled.
Baseline demographics (mean age, mean weight, mean BMI) were not significantly different in both groups. Moreover, no significant difference was seen in terms of other variables such as anthropometric indices, body composition, stage of fatty liver, 25(OH)D
3 level, sun exposure and physical activity level between the 2 groups at the beginning of the study. The characteristics of the participants are shown in
Table 1. No statistically significant differences in energy and nutrient intake were observed either within or between groups before and after the intervention. The intake of vitamin D was significantly different between the 2 groups at baseline current (p = 0.03) (
Table 2).
Table 1 Main characteristics of participants with nonalcoholic fatty liver disease
Table 1
|
Variable |
Group A |
Group B |
p value*
|
|
Age (yr) |
40.39 ± 6.22 |
39.91 ± 7.16 |
0.740 |
|
Sex (male) |
52.2 |
54.5 |
0.830 |
|
Weight (kg) |
79.59 ± 9.60 |
81.24 ± 12.59 |
0.490 |
|
BMI (kg/m2) |
28.38 ± 3.09 |
28.45 ± 2.43 |
0.900 |
|
WC (cm) |
96.11 ± 9.05 |
96.13 ± 7.96 |
0.643 |
|
WHR |
0.89 ± 0.48 |
0.90 ± 0.05 |
0.280 |
|
BFM (kg) |
24.49 ± 5.96 |
25.24 ± 5.11 |
0.138 |
|
LBM (kg) |
55.25 ± 9.55 |
55.98 ± 11.86 |
0.061 |
|
25(OH)D3 (ng/mL) |
24.14 ± 15.31 |
20.66 ± 11.45 |
0.231 |
|
Sun exposure (min/day) |
78.05 ± 99.89 |
39.32 ± 52.28 |
0.087 |
|
PAL (MET/h/week) |
|
|
|
|
Light |
20.5 |
27.3 |
0.065 |
|
Moderate |
54.5 |
65.9 |
- |
|
Intense |
25.0 |
6.8 |
- |
Table 2 Energy and nutrients intake of patients with nonalcoholic fatty liver disease
Table 2
|
Variable |
Group A |
Group B |
P2 |
P3 |
|
Before |
After |
P1 |
Before |
After |
P1 |
|
Energy (kcal/day) |
2,195.81 ± 716.09 |
2,213.17 ± 646.94 |
0.82 |
2,301.06 ± 803.69 |
2,294.91 ± 845.35 |
0.93 |
0.51 |
0.61 |
|
Carbohydrate (g/day) |
328.29 ± 102.75 |
336.12 ± 108.65 |
0.55 |
340.15 ± 124.74 |
330.63 ± 117.67 |
0.40 |
0.62 |
0.82 |
|
Protein (g/day) |
73.90 ± 23.97 |
79.15 ± 25.79 |
0.13 |
84.44 ± 36.70 |
87.79 ± 38.52 |
0.34 |
0.11 |
0.22 |
|
Fat (g/day) |
69.20 ± 34.08 |
64.74 ± 21.57 |
0.27 |
70.06 ± 29.83 |
71.84 ± 33.44 |
0.67 |
0.90 |
0.24 |
|
Vitamin D (IU/day) |
15.92 ± 25.20 |
18.72 ± 30.40 |
0.73 |
28.88 ± 32.40 |
26.08 ± 41.60 |
0.70 |
0.03*
|
0.27 |
After the intervention, no significantly different was observed in both groups between the mean of weight, BMI, WC, WHR, and LBM, compared to baseline. Both groups had a decrease in body fat mass (BFM); group B had a significant decrease (p = 0.01), whereas group A had a non-significant decrease in BFM (p = 0.058) (
Table 3).
Table 3 Serum 25(OH)D3, glycemic indices and anthropometric indices of patients with nonalcoholic fatty liver disease
Table 3
|
Variable |
Group A |
Group B |
P2 |
P3 |
|
Before |
After |
P1 |
Before |
After |
P1 |
|
25(OH)D3 (ng/mL) |
24.14 ± 15.31 |
30.12 ± 10.64 |
< 0.001*
|
20.66 ± 11.45 |
20.45 ± 10.97 |
0.861 |
0.231 |
0.001†
|
|
FBS (mg/dL) |
102.29 ± 10.59 |
90.36 ± 9.66 |
< 0.001*
|
100.04 ± 12.16 |
91.54 ± 15.74 |
< 0.001*
|
0.35 |
0.91 |
|
Insulin (micro U/mL) |
17.56 ± 10.04 |
13.40 ± 5.70 |
0.003*
|
15.69 ± 9.35 |
13.17 ± 5.01 |
0.06 |
0.28 |
0.84 |
|
HOMA-IR |
4.51 ± 2.65 |
2.98 ± 1.24 |
< 0.001 |
3.94 ± 2.53 |
3.01 ± 1.37 |
0.013 |
0.30 |
0.92 |
|
Weight (kg) |
79.59 ± 9.60 |
79.21 ± 9.49 |
0.254 |
81.23 ± 12.59 |
80.91 ± 12.59 |
0.216 |
0.49 |
0.47 |
|
BMI (kg/m2) |
28.38 ± 3.09 |
28.23 ± 2.94 |
0.191 |
28.45 ± 2.44 |
28.34 ± 2.49 |
0.238 |
0.90 |
0.84 |
|
Body fat (kg) |
24.49 ± 5.96 |
23.88 ± 5.09 |
0.058 |
25.24 ± 5.11 |
24.62 ± 5.24 |
0.010*
|
0.52 |
0.50 |
|
WC (cm) |
96.11 ± 9.05 |
95.21 ± 8.79 |
0.267 |
96.13 ± 7.96 |
96.59 ± 7.52 |
0.535 |
0.99 |
0.43 |
|
WHR |
0.89 ± 0.05 |
0.889 ± 0.05 |
0.267 |
0.91 ± 0.06 |
0.900 ± 0.06 |
0.089 |
0.28 |
0.33 |
|
LBM (kg) |
55.25 ± 9.55 |
55.33 ± 9.59 |
0.682 |
55.98 ± 11.86 |
56.28 ± 12.11 |
0.147 |
0.75 |
0.99 |
|
SBP (mmHg) |
121.14 ± 13.58 |
117.73 ± 12.20 |
0.041*
|
121.50 ± 12.10 |
116.34 ± 11.48 |
0.008*
|
0.89 |
0.58 |
|
DBP (mmHg) |
78.82 ± 8.74 |
77.07 ± 8.71 |
0.234 |
79.91 ± 11.22 |
78.36 ± 8.53 |
0.330 |
0.61 |
0.48 |
|
Sun exposure (min) |
78.05 ± 99.89 |
68.30 ± 77.80 |
0.512 |
39.32 ± 52.28 |
50.91 ± 61.93 |
0.015*
|
0.08 |
0.71 |
|
Physical activity(MET/hr/wk) |
2,565.05 ± 4,867.90 |
2,210.86 ± 3,898.62 |
0.97 |
946.17 ± 1,035.60 |
861.28 ± 1,133.55 |
0.17 |
0.12 |
0.10 |
Although DBP did not show any significant change after the intervention, the level of SBP decreased significantly in both groups (group A: p = 0.041, group B: p = 0.008). Physical activity and sun exposure showed no significant changes in both groups.
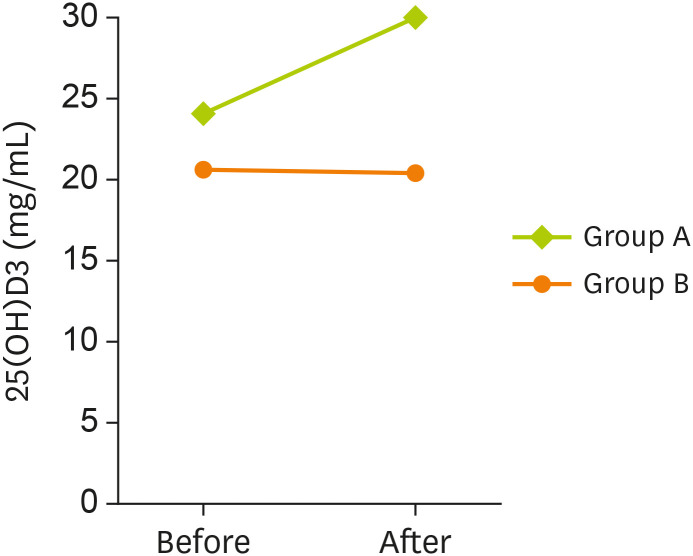
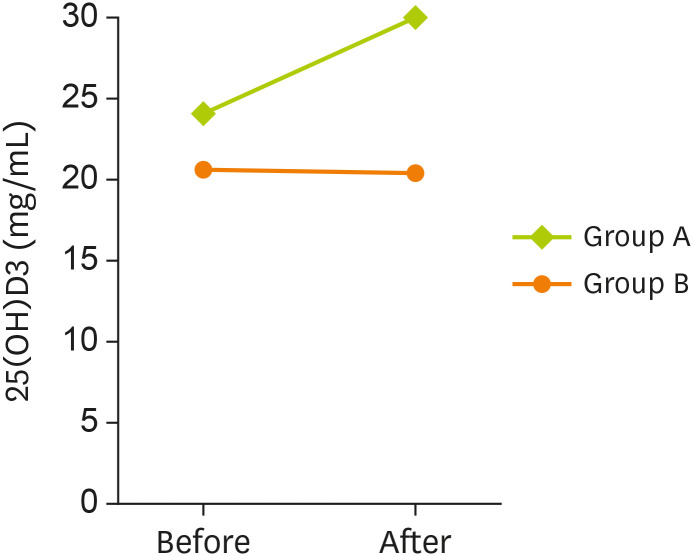
At baseline, serum levels of 25(OH)D
3 did not show any significant difference between the 2 groups. As shown in
Table 3, after 3 months of intervention, a significant increase was observed in serum levels of 25(OH)D
3 in group A (p < 0.001), but no changes were found in group B (p = 0.861). After the intervention, serum levels of 25(OH)D
3 increased significantly in group A, compared to group B (p < 0.001) (
Figure 2).
Figure 2
Serum level of 25(OH)D3 among 2 studied groups in before and after intervention.
Data are represented as below: group A, probiotic-vitamin D fortified yogurt; B, unfortified yogurt.
*A p value less than 0.05 is considered statistically significant.
Levels of glycemic indices (FBS, insulin and HOMA-IR) at baseline showed no significant differences between the groups, but after the intervention, levels of FBS and HOMA-IR decreased significantly in both groups (p < 0.001). A significant reduction in insulin level was seen only in group A (p = 0.003).
DISCUSSION
In the current trial, we investigated the effect of vitamin D and probiotic fortified yogurt consumption for 12 weeks in patients with NAFLD on serum levels of 25(OH)D
3 metabolic responses and glycemic indices compared to regular yogurt. Our data indicated that vitamin D and probiotic co-supplementation cause significant improvements in the levels of 25(OH)D
3, and insulin in patients with NAFLD. However, our result indicated that vitamin D plus probiotic fortified yogurt did not significantly affect insulin resistance and glucose levels between intervention and control groups. To the best of our knowledge, this is the first randomized clinical trial that investigated the effect of vitamin D and probiotic fortified food in NAFLD patients. Yogurt has been used to increased nutrients intake in several studies [
35,
36,
37]. In line with our results, Jafari et al. [
36] show that vitamin D fortified yogurt can significantly increase levels of 25(OH)D
3. In addition, it has been shown that vitamin D–fortified yogurt can improve lipids and lipoproteins [
38]. Vitamin D status has a significant association with NAFLD pathophysiology [
39], and vitamin D deficiency is associated with an increased prevalence of NAFLD, so correcting vitamin D status could be very useful to management of NAFLD complications [
29]. Besides, although the precise molecular function of probiotics in several diseases have not been determined yet, lots of their impacts have elucidated to be useful in NAFLD, such as the regulation of the intestinal bacterium profile, generation of antibacterial materials, ameliorated epithelial barrier function and decreased gastrointestinal inflammation [
40]. Besides probiotics have anti-inflammatory and antioxidant proprieties [
41], and also have several beneficial impacts on metabolic parameters [
42,
43].
Our results indicated that vitamin D and probiotics co-supplementation as an enriched yogurt did not have any significant effects on glycemic parameters compared to the control group in NAFLD patients. These results are in contrast with Jamilian et al. [
44] that showed that 6 weeks vitamin D and probiotic co-supplementation in gestational diabetes mellitus (GDM) patients could significantly decrease fasting blood glucose (p < 0.001), serum insulin levels (p = 0.001) and HOMA-IR (p < 0.001), and significantly elevated the quantitative insulin-sensitivity check index (QUICKI; p = 0.001) compared with the control. Possible reasons for this inconsistency between our results and Jamilian's is maybe because of different pathophysiology of GDM and NAFLD patients. Besides, different baseline of glycemic parameters, which is higher in GDM patients, the different results in our study compare to Jamilian et al. [
44]. In another study Raygan et al. [
45] demonstrated that compared with the control, vitamin D and probiotic co-supplementation leads to a significant decrease in serum insulin levels (p = 0.009), HOMA-IR (p = 0.02), and a significant elevation the QUICKI (p = 0.003) in type 2 diabetes patients. Different in baseline levels of insulin in diabetic and NAFLD patients may cause this inconsistency of results [
46]. However, in line with our results, Raygan et al. [
45] also demonstrated that vitamin D and probiotic co-supplementation leads to a significant increase in serum 25-OH-vitamin D (p < 0.001).
Vitamin D deficiency is related to metabolic complications in NAFLD [
47]. So, vitamin D supplementation could be beneficial in the prevention and management of this disease.
Oxidative stress is thought to play a critical role in the pathogenesis of several metabolic disorders [
48]. Oxidative stress and inflammation play a pivotal role in the pathophysiology of NAFLD [
49,
50]. Moreover, vitamin D has potent anti-inflammatory [
51] and antioxidant proprieties [
18,
52]. So, it seems that vitamin D and probiotic co-supplementation could increase synergistically antioxidant and anti-inflammatory, and improve body defense against oxidant and inflammatory mediators [
53]. Furthermore because of other health benefits of yogurt as a good source of calcium and protein [
54], it seems that enrichment of yogurt is an excellent way to deliver exciting nutrients and supplements to the host.
The present trial had some limitations. We did not determine fecal bacteria loads at the beginning and end of the intervention and characterize the bacterial profile of intestine before and after probiotic intake. Moreover, we did not assess the impacts of vitamin D and probiotic co-supplementation on the expression of genes associated with glycemic parameters and inflammation in NAFLD patients. Also, we did not evaluate the sun exposure of participants as a determinant factor in serum 25-OH-vitamin D levels.
CONCLUSION
Overall, vitamin D and probiotic co-supplementation in enriched yogurt after 12 weeks among NAFLD patients had beneficial impacts on serum 25-OH-vitamin D levels, but did not affect glycemic parameters and blood pressures. More substantial well-designed randomized clinical trials still needed to draw a specific conclusion about the effect of vitamin D and probiotic co-supplementation on NAFLD patients.
Kermanshah University of Medical Scienceshttps://doi.org/10.13039/50110000531796621
NOTES
-
Funding: The Research Council approved this study of Kermanshah University of Medical Sciences (grant No. 96621).
-
Conflict of Interest: The authors declare that they have no competing interests.
ACKNOWLEDGEMENTS
We wish to thank and acknowledge the valuable contribution of all participants in this study and especially want to acknowledge the Research Council.
This paper is a portion of a thesis in partial fulfillment of the requirements for the degree of Master of Science (M.Sc.) in Nutrition, Faculty of Nutrition and Food Sciences, Kermanshah University of Medical Sciences, Kermanshah, Iran.
REFERENCES
- 1. Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol 2010;5:145-171.
- 2. Petta S, Gastaldelli A, Rebelos E, Bugianesi E, Messa P, Miele L, Svegliati-Baroni G, Valenti L, Bonino F. Pathophysiology of non alcoholic fatty liver disease. Int J Mol Sci 2016;17:E2082.
- 3. Pasdar Y, Moradi S, Moludi J, Darbandi M, Niazi P, Nachvak SM, Abdollahzad H. Risk of metabolic syndrome in non-alcoholic fatty liver disease patients. Med J Nutrition Metab 2019;12:1-11.
- 4. Kelishadi R, Poursafa P. Obesity and air pollution: global risk factors for pediatric non-alcoholic fatty liver disease. Hepat Mon 2011;11:794-802.
- 5. Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 2006;43:S99-112.
- 6. Wong VW. Nonalcoholic fatty liver disease in Asia: a story of growth. J Gastroenterol Hepatol 2013;28:18-23.
- 7. Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to treatment. Frontline Gastroenterol 2014;5:277-286.
- 8. Mollahosseini M, Daneshzad E, Rahimi MH, Yekaninejad MS, Maghbooli Z, Mirzaei K. The association between fruit and vegetable intake and liver enzymes (aspartate and alanine transaminases) in Tehran, Iran. Ethiop J Health Sci 2017;27:401-410.
- 9. Asrih M, Jornayvaz FR. Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J Endocrinol 2013;218:R25-36.
- 10. Vafa M, Heshmati J, Sadeghi H, Shidfar F, Namazi N, Baradaran H, Heydarpour B, Jalili Z. Is exclusive breastfeeding and its duration related to cardio respiratory fitness in childhood? J Matern Fetal Neonatal Med 2016;29:461-465.
- 11. Javed I, Sarfraz M, Muhammad F, Aslam B, Rahman ZU, Khan MZ, Khaliq T, Khan FH, Ahmad M. Lipid lowering effect of a herbal mixture in hyperlipidaemic adult male albino mice. Pak Vet J 2014;34:489-493.
- 12. Yoo SR, Kim YJ, Park DY, Jung UJ, Jeon SM, Ahn YT, Huh CS, McGregor R, Choi MS. Probiotics L. plantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-induced obesity. Obesity (Silver Spring) 2013;21:2571-2578.
- 13. Kazemi A, Soltani S, Ghorabi S, Keshtkar A, Daneshzad E, Nasri F, Mazloomi SM. Effect of probiotic and synbiotic supplementation on inflammatory markers in health and disease status: a systematic review and meta-analysis of clinical trials. Clin Nutr 2020;39:789-819.
- 14. Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014;146:1513-1524.
- 15. Jazayeri-Tehrani SA, Rezayat SM, Mansouri S, Qorbani M, Alavian SM, Daneshi-Maskooni M, Hosseinzadeh-Attar MJ. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): a double-blind randomized placebo-controlled clinical trial. Nutr Metab (Lond) 2019;16:8.
- 16. Economou V, Gousia P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect Drug Resist 2015;8:49-61.
- 17. Kulda V. Vitamin D metabolism. Vnitr Lek 2012;58:400-404.
- 18. Sepidarkish M, Farsi F, Akbari-Fakhrabadi M, Namazi N, Almasi-Hashiani A, Maleki Hagiagha A, Heshmati J. The effect of vitamin D supplementation on oxidative stress parameters: a systematic review and meta-analysis of clinical trials. Pharmacol Res 2019;139:141-152.
- 19. Verrusio W, Andreozzi P, Renzi A, Musumeci M, Gueli N, Cacciafesta M. Association between serum vitamin D and metabolic syndrome in middle-aged and older adults and role of supplementation therapy with vitamin D. Ann Ist Super Sanita 2017;53:54-59.
- 20. Alipour S, Saberi A, Seifollahi A, Shirzad N, Hosseini L. Risk factors and prevalence of vitamin d deficiency among Iranian women attending two university hospitals. Iran Red Crescent Med J 2014;16:e15461.
- 21. Kaykhaei MA, Hashemi M, Narouie B, Shikhzadeh A, Rashidi H, Moulaei N, Ghavami S. High prevalence of vitamin D deficiency in Zahedan, southeast Iran. Ann Nutr Metab 2011;58:37-41.
- 22. Hovsepian S, Amini M, Aminorroaya A, Amini P, Iraj B. Prevalence of vitamin D deficiency among adult population of Isfahan City, Iran. J Health Popul Nutr 2011;29:149-155.
- 23. Shahla A, Charehsaz S, Talebi R, Omrani M. Vitamin D deficiency in young females with musculoskeletal complaints in Urmia, northwest of Iran. Iran J Med Sci 2015;30:88-90.
- 24. Barchetta I, Angelico F, Del Ben M, Baroni MG, Pozzilli P, Morini S, Cavallo MG. Strong association between non alcoholic fatty liver disease (NAFLD) and low 25(OH) vitamin D levels in an adult population with normal serum liver enzymes. BMC Med 2011;9:85.
- 25. Jafari T, Askari G, Mirlohi M, Javanmard SH, Faghihimani E, Fallah AA. Stability of vitamin D3 in fortified yoghurt and yoghurt drink (Doogh). Adv Biomed Res 2016;5:52.
- 26. Nabavi S, Rafraf M, Somi MH, Homayouni-Rad A, Asghari-Jafarabadi M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci 2014;97:7386-7393.
- 27. Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P; CONSORT Group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295-309.
- 28. Greenblatt DJ. Elimination half-life of drugs: value and limitations. Annu Rev Med 1985;36:421-427.
- 29. Eliades M, Spyrou E. Vitamin D: a new player in non-alcoholic fatty liver disease? World J Gastroenterol 2015;21:1718-1727.
- 30. Mostafai R, Mohammadi R, Mostafa Nachvak S, Rezaei M, Pasdar Y, Abdollahzad H, Rezvanmadani F, Moradi S, Morvaridzadeh M, Niazi P, Adeli K. Fortified yogurt with vitamin D as a cost-effective food to prevent diabetes: a randomized double-blind clinical trial. J Funct Foods 2018;42:137-145.
- 31. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911-1930.
- 32. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412-419.
- 33. Ghaffarpour M, Houshiar-Rad A, Kianfar H. The manual for household measures, cooking yields factors and edible portion of foods, Tehran. Nashre Olume Keshavarzy 1999;7:213.
- 34. Fesharaki MGh, Azad E. Evaluation of the reliability and validity of Azad-Fesharaki's physical activity questionnaire (AFPAQ). Arak Med Univ J 2011;14:36-44.
- 35. Nikooyeh B, Neyestani TR, Farvid M, Alavi-Majd H, Houshiarrad A, Kalayi A, Shariatzadeh N, Gharavi A, Heravifard S, Tayebinejad N, Salekzamani S, Zahedirad M. Daily consumption of vitamin D- or vitamin D + calcium-fortified yogurt drink improved glycemic control in patients with type 2 diabetes: a randomized clinical trial. Am J Clin Nutr 2011;93:764-771.
- 36. Jafari T, Faghihimani E, Feizi A, Iraj B, Javanmard SH, Esmaillzadeh A, Fallah AA, Askari G. Effects of vitamin D-fortified low fat yogurt on glycemic status, anthropometric indexes, inflammation, and bone turnover in diabetic postmenopausal women: a randomised controlled clinical trial. Clin Nutr 2016;35:67-76.
- 37. Mostafai R, Nachvakc SM, Mohammadi R, Rocha RS, da Silva MC, Esmerino EA, Nascimento KO, Cruz AG, Mortazavian AM. Effects of vitamin D-fortified yogurt in comparison to oral vitamin D supplement on hyperlipidemia in pre-diabetic patients: a randomized clinical trial. J Funct Foods 2019;52:116-120.
- 38. Heravifard S, Neyestani TR, Nikooyeh B, Alavi-Majd H, Houshiarrad A, Kalayi A, Shariatzadeh N, Zahedirad M, Tayebinejad N, Salekzamani S, Khalaji N, Gharavi A. Regular consumption of both vitamin D- and calcium- and vitamin D-fortified yogurt drink is equally accompanied by lowered blood lipoprotein (a) and elevated apoprotein A1 in subjects with type 2 diabetes: a randomized clinical trial. J Am Coll Nutr 2013;32:26-30.
- 39. Kwok RM, Torres DM, Harrison SA. Vitamin D and nonalcoholic fatty liver disease (NAFLD): is it more than just an association? Hepatology 2013;58:1166-1174.
- 40. Iacono A, Raso GM, Canani RB, Calignano A, Meli R. Probiotics as an emerging therapeutic strategy to treat NAFLD: focus on molecular and biochemical mechanisms. J Nutr Biochem 2011;22:699-711.
- 41. Heshmati J, Farsi F, Shokri F, Rezaeinejad M, Almasi-Hashiani A, Vesali S, Sepidarkish M. A systematic review and meta-analysis of the probiotics and synbiotics effects on oxidative stress. J Funct Foods 2018;46:66-84.
- 42. Heshmati J, Farsi F, Yosaee S, Razavi M, Rezaeinejad M, Karimie E, Sepidarkish M. The effects of probiotics or synbiotics supplementation in women with polycystic ovarian syndrome: a systematic review and meta-analysis of randomized clinical trials. Probiotics Antimicrob Proteins 2019;11:1236-1247.
- 43. Agah S, Akbari A, Heshmati J, Sepidarkish M, Morvaridzadeh M, Adibi P, Mazidi M, Farsi F, Ofori-Asenso R, Talley NJ, Feinle-Bisset C. Systematic review with meta-analysis: effects of probiotic supplementation on symptoms in functional dyspepsia. J Funct Foods 2020;68:103902.
- 44. Jamilian M, Amirani E, Asemi Z. The effects of vitamin D and probiotic co-supplementation on glucose homeostasis, inflammation, oxidative stress and pregnancy outcomes in gestational diabetes: a randomized, double-blind, placebo-controlled trial. Clin Nutr 2019;38:2098-2105.
- 45. Raygan F, Ostadmohammadi V, Bahmani F, Asemi Z. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry 2018;84:50-55.
- 46. Namazi N, Khodamoradi K, Khamechi SP, Heshmati J, Ayati MH, Larijani B. The impact of cinnamon on anthropometric indices and glycemic status in patients with type 2 diabetes: a systematic review and meta-analysis of clinical trials. Complement Ther Med 2019;43:92-101.
- 47. Wang X, Li W, Zhang Y, Yang Y, Qin G. Association between vitamin D and non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: results from a meta-analysis. Int J Clin Exp Med 2015;8:17221-17234.
- 48. Darvish Damavandi R, Mousavi SN, Shidfar F, Mohammadi V, Rajab A, Hosseini S, Heshmati J. Effects of daily consumption of cashews on oxidative stress and atherogenic indices in patients with type 2 diabetes: a randomized, controlled-feeding trial. Int J Endocrinol Metab 2019;17:e70744.
- 49. Albano E, Mottaran E, Occhino G, Reale E, Vidali M. Review article: role of oxidative stress in the progression of non-alcoholic steatosis. Aliment Pharmacol Ther 2005;22(Suppl 2):71-73.
- 50. Sepidarkish M, Akbari-Fakhrabadi M, Daneshzad E, Yavari M, Rezaeinejad M, Morvaridzadeh M, Heshmati J. Effect of omega-3 fatty acid plus vitamin E Co-Supplementation on oxidative stress parameters: a systematic review and meta-analysis. Clin Nutr 2020;39:1019-1025.
- 51. Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol 2011;51:311-336.
- 52. Fazelian S, Hoseini M, Namazi N, Heshmati J, Sepidar Kish M, Mirfatahi M, Some Olia AS. Effects of L-arginine supplementation on antioxidant status and body composition in obese patients with pre-diabetes: a randomized controlled clinical trial. Adv Pharm Bull 2014;4:449-454.
- 53. Ardeshirlarijani E, Tabatabaei-Malazy O, Mohseni S, Qorbani M, Larijani B, Baradar Jalili R. Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: a meta-analysis of randomized trials. Daru 2019;27:827-837.
- 54. Heshmati J, Sepidarkish M, Namazi N, Shokri F, Yavari M, Fazelian S, Khorshidi M, Shidfar F. Impact of dietary calcium supplement on circulating lipoprotein concentrations and atherogenic indices in overweight and obese individuals: a systematic review. J Diet Suppl 2019;16:357-367.
 , Seyed Mostafa Nachvak2
, Seyed Mostafa Nachvak2 , Reza Mohammadi3
, Reza Mohammadi3 , Shima Moradi2
, Shima Moradi2 , Roghayeh Mostafai1
, Roghayeh Mostafai1 , Ana Beatriz Pizarro4
, Ana Beatriz Pizarro4 , Hadi Abdollahzad2
, Hadi Abdollahzad2